SENNA-S- docusate sodium, sennosides tablet, film coated
Senna-S by
Drug Labeling and Warnings
Senna-S by is a Otc medication manufactured, distributed, or labeled by Granulation Technology, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredients (in each tablet)
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Stop using and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
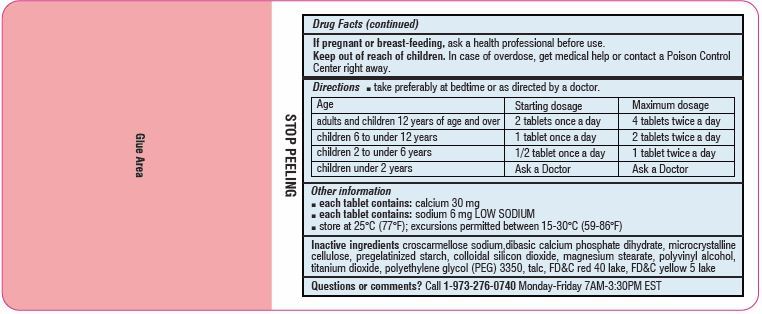
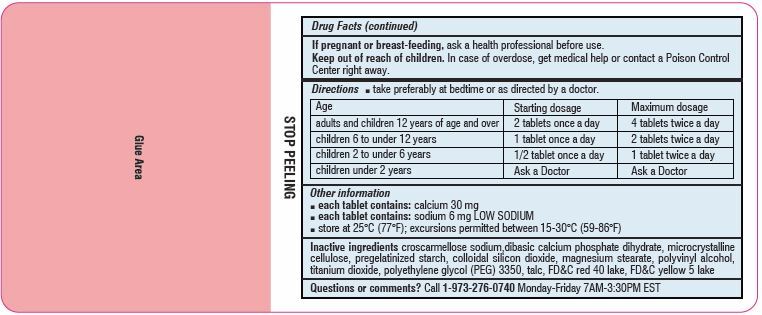
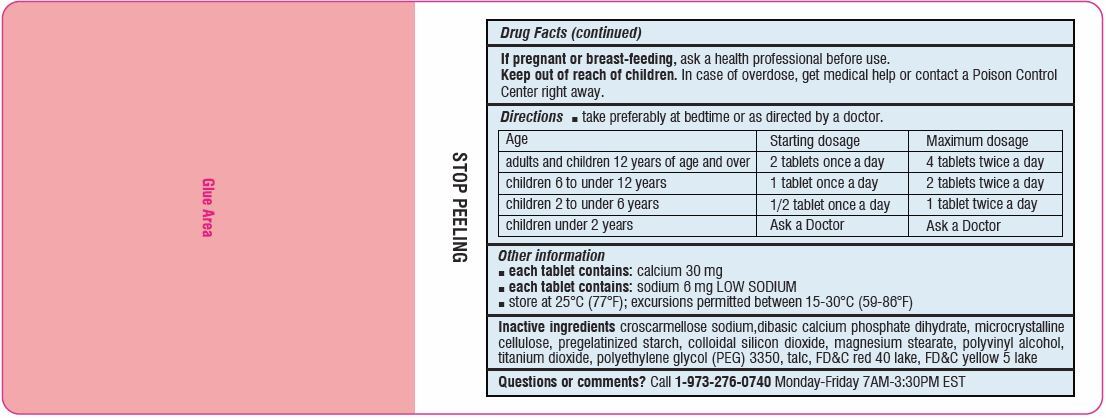
Directions
- Take preferably at bedtime or as directed by a doctor
age starting dosage maximum dosage adults and children
12 years of age and over2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor - Other information
- Inactive ingredients
-
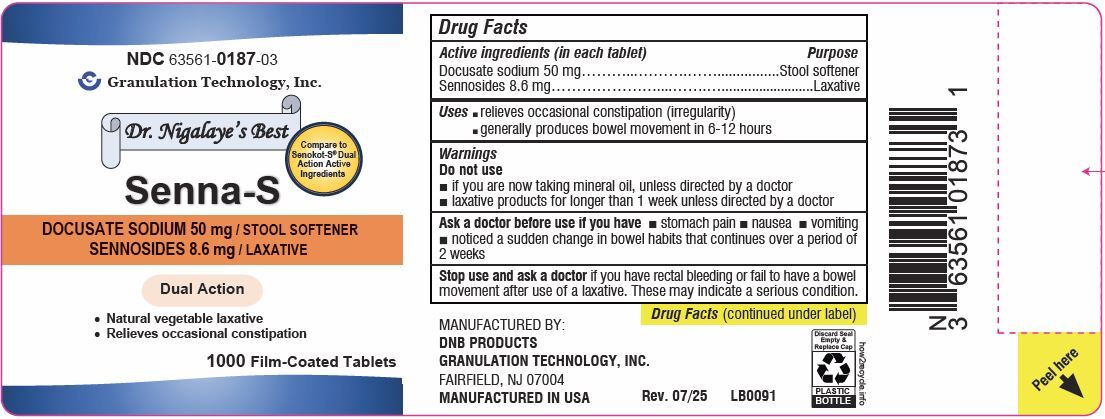
Principal Display Panel
NDC: 63561-0187-01
Senna-S
DOCUSATE SODIUM 50 mg / STOOL SOFTENER
SENNOSIDES 8.6 mg / LAXATIVE
Compare to Senokot-S ®Dual Action Active Ingredients
100 Film-Coated Tablets

NDC: 63561-0187-02
Senna-S
DOCUSATE SODIUM 50 mg / STOOL SOFTENER
SENNOSIDES 8.6 mg / LAXATIVE
Compare to Senokot-S ®Dual Action Active Ingredients
220 Film-Coated Tablets

NDC: 63561-0187-03
Senna-S
DOCUSATE SODIUM 50 mg / STOOL SOFTENER
SENNOSIDES 8.6 mg / LAXATIVE
Compare to Senokot-S ®Dual Action Active Ingredients
1000 Film-Coated Tablets
-
INGREDIENTS AND APPEARANCE
SENNA-S
docusate sodium, sennosides tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63561-0187 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) MICROCRYSTALLINE CELLULOSE 200 (UNII: 5XDI2TS1EZ) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) TALC (UNII: 7SEV7J4R1U) FD&C RED NO. 40 ALUMINUM LAKE (UNII: 6T47AS764T) FD&C YELLOW NO. 5 ALUMINUM LAKE (UNII: JQ6BLH9FR7) Product Characteristics Color orange Score no score Shape ROUND Size 10mm Flavor Imprint Code G187 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63561-0187-2 220 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/29/2025 2 NDC: 63561-0187-1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/29/2025 3 NDC: 63561-0187-3 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/29/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/29/2025 Labeler - Granulation Technology, Inc. (847132193) Registrant - Granulation Technology, Inc. (847132193) Establishment Name Address ID/FEI Business Operations Granulation Technology, Inc. 847132193 manufacture(63561-0187)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.