ALFA VETERINARY 0.9% SODIUM CHLORIDE- sodium chloride injection, solution injection, solution
Alfa Veterinary 0.9% Sodium Chloride by
Drug Labeling and Warnings
Alfa Veterinary 0.9% Sodium Chloride by is a Animal medication manufactured, distributed, or labeled by Laboratorios Alfa SRL. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
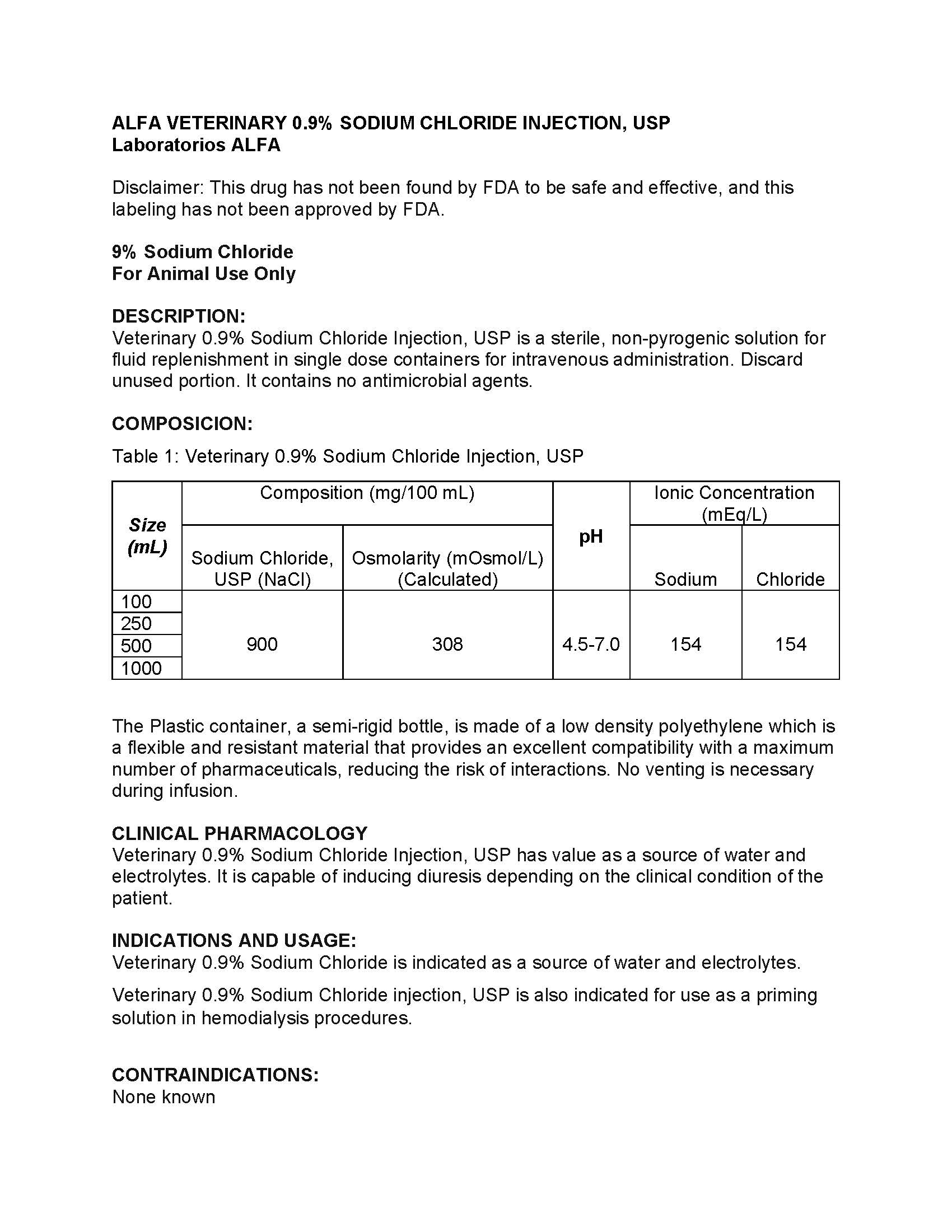
DESCRIPTION
Veterinary 0.9% Sodium Chloride Injection, USP is a sterile, non-pyrogenic solution for fluid replenishment in single dose containers for intravenous administration. Discard unused portion. It contains no antimicrobial agents.
Table 1: Veterinary 0.9% Sodium Chloride Injection, USP Size (mL) Compostion(mg/100 mL)
pH Ionic Concentration (mEq/L) Sodium Chloride,
USP (NaCl)
Osmolarity (mOsmol/L)
(Calculated)Sodium Chloride 100 900 308 4.5-7.0 154 154 250 500 1000 The Plastic container, a semi-rigid bottle, is made of a low density polyethylene which is a flexible and resistant material that provides an excellent compatibility with a maximum number of pharmaceuticals, reducing the risk of interactions. No venting is necessary during infusion.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Caution must be exercised in the administration of veterinary 0.9% Sodium Chloride Injection, USP to patients receiving corticosteroids or corticotrophin.
Do not administer unless solution is clear, and seal is intact.
-
DOSAGE & ADMINISTRATION
As directed by a veterinarian. Dosage is dependent upon the age, weight and clinical condition of the patient, as well as laboratory determinations.
Parenteral drug products should be inspected visually for particulate matter and discolorations prior to administration whenever solution and container permit.
All injections in plastic containers are intended for intravenous administration using sterile equipment.
Additives may be incompatible. Complete information is not available. Those additives known to be incompatible should not be used.
Consult with pharmacist, if available. If, in the informed judgment of the veterinarian, it is deemed advisable to introduce additives, use aseptic technique. Mix thoroughly when additives have been introduced.
Do not store solutions containing additives. Discard unused portion.
Overdosage
In an event of over hydration or solute overload, re-evaluate the patient and institute appropriate corrective measures. See Warnings, Precautions and Adverse Reactions.
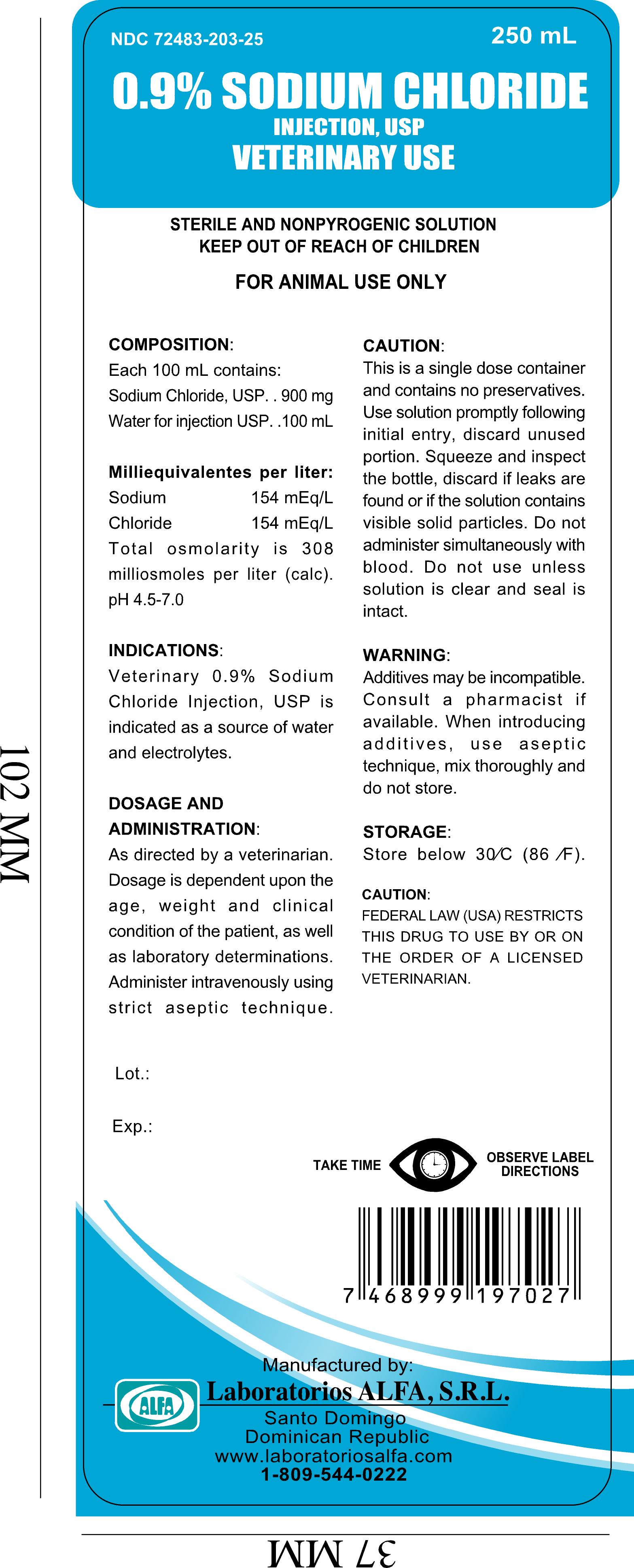
Packaging NDC: 72483-203-10 1000 mL in Plastic Bottle; NDC: 72483-203-05 500 mL in Plastic Bottle; NDC: 72483-203-25 250 mL in Plastic Bottle; NDC: 72483-203-01 100 mL in Plastic Bottle
NDC: 72483-203-10 1000 mL in Plastic Bottle
NDC: 72483-203-05 500 mL in Plastic Bottle
NDC: 72483-203-25 250 mL in Plastic Bottle
NDC: 72483-203-01 100 mL in Plastic Bottle
DIRECTIONS FOR USE PLASTIC CONTAINER
Preparation and administration (Use Aspetic Technique):
- Check for minute leaks by squeezing the container firmly. If leaks are found discard solutions as sterility may be impaired.
- Suspend container from eyelet support.
- Remove Plastic protector from port area at bottom of container.
- Hold bottle in vertical position and insert IV administration set in outlet port.
To add Medication:
WARNING: Additives may be incompatible.
- Prepare medication site.
- Using syringe with 19 to 22 gauge needle, puncture inlet port and inject.
- Mix solution and medication thoroughly. Return container to in-use position and continue administration.
- OVERDOSAGE
- STORAGE
-
PRECAUTION FOR USE OF THE BOTTLE
This is a single dose container and does not contain preservatives.
Use the solution immediately after the bottle is opened, discard the remaining one.
Squeeze and inspect the bottle, discard if leaks are found or if the solution contains visible and solid particles.Do not administer simultaneously with blood.
Do not use it unless solution is clear and seal is intact, the solution containing dextrose may be contraindicated in patients with a known allergy to corn or corn products.DIRECTIONS FOR USE PLASTIC CONTAINER
Preparation and administration
- Check for minute leaks by squeezing the container firmly. If leaks are found, discard solution as sterility may be impaired.
- Suspend container from eyelet support.
- Remove Plastic protector from ports area at the bottom of container.
- Hold the bottle in vertical position and inset pyrogen free IV administration set in the outlet port. Use aseptic technique.
To Add Medication
WARNING:Additives may be incompatible.
To add medication before solution administration.- Prepare medication site.
- Using syringe with 19 to 22 gauge needle, puncture inlet port and inject.
- Mix solution and medication thoroughly. Return container to in-use position and continue administration. For high density medication such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
To add medication during solution administration
- Close clamp on the set.
- Prepare medication site.
- Using syringe with 19 to 22 gauge needle, puncture inlet port and inject.
- Remove container from IV pole and/or turn to an upright position.
- Mix solution and medication thoroughly.
- Return container to in use position and continue administration.
- PACKAGE INSERT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALFA VETERINARY 0.9% SODIUM CHLORIDE
sodium chloride injection, solution injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 72483-203 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 900 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72483-203-10 1000 mL in 1 BOTTLE, PLASTIC 2 NDC: 72483-203-05 500 mL in 1 BOTTLE, PLASTIC 3 NDC: 72483-203-25 250 mL in 1 BOTTLE, PLASTIC 4 NDC: 72483-203-01 100 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/04/2019 Labeler - Laboratorios Alfa, S.R.L. (815941244)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.