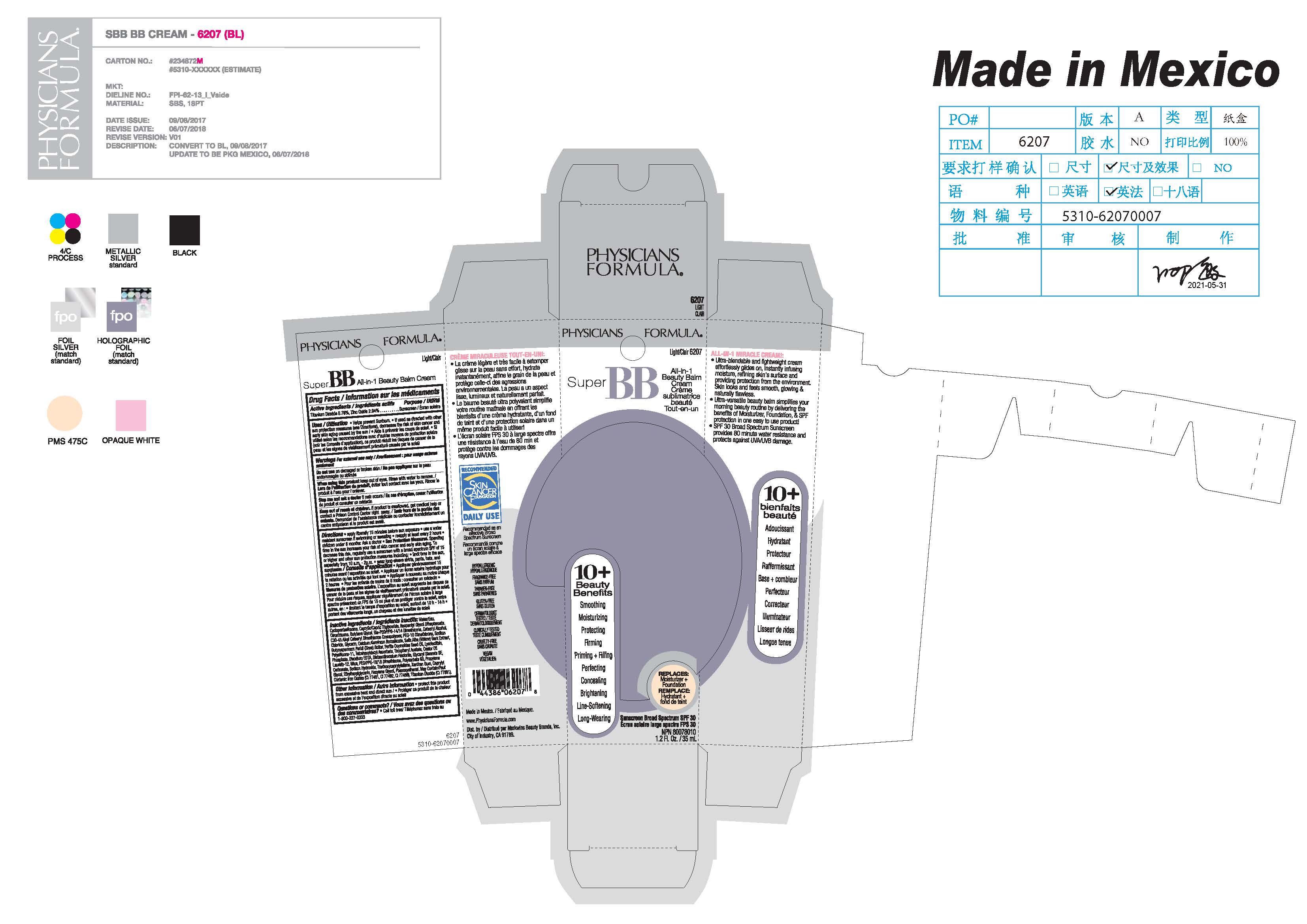
Super BB Cream Beauty Balm Cream 6207, 7867; 69514-308
Super BB All in 1 Beauty Balm Cream by
Drug Labeling and Warnings
Super BB All in 1 Beauty Balm Cream by is a Otc medication manufactured, distributed, or labeled by Markwins Beauty Brands. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SUPER BB ALL IN 1 BEAUTY BALM CREAM- titanium dioxide, zinc oxide cream
Markwins Beauty Brands
----------
Super BB Cream Beauty Balm Cream 6207, 7867; 69514-308
Helps prevent Sunburn. If used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early aging caused by the sun
For external use only
Do not use on damage or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Directions
apply liberally 15 minutes before sun exposure
reapply at least every 2 hours
children under 6 months of age: Ask a doctor
use a water resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this, risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun , especially from 10 a.m. - 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
Water/Eau, Cyclopentasiloxane, Caprylic/Capric Triglyceride, Neopentyl Glycol Diheptanoate, Dimethicone, Butylene Glycol, Bis-PEG/PPG-14/14 Dimethicone, Cetearyl Alcohol, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, PEG-10 Dimethicone, Sodium Chloride, Glycerin, Calcium Aluminum Borosilicate, Salix Alba (Willow) Bark Extract, Butyrospermum Parkii (Shea) Butter, Perilla Ocymoides Seed Oil, Lysolecithin, Polysilicone-11, Tetrahexyldecyl Ascorbate, Tocopheryl Acetate, Castor Oil Phosphate, Disodium EDTA, Disteardimonium Hectorite, Glyceryl Stearate SE, Laureth-12, Mica, PEG/PPG-18/18 Dimethicone, Polysorbate 60, Propylene Carbonate, Sodium Hydroxide, Triethoxycaprylylsilane, Xanthan Gum, Caprylyl Glycol, Ethylhexylglycerin, Hexylene Glycol, Phenoxyethanol. May Contain/Peut Contenir: Iron Oxides (CI 77491, CI 77492, CI 77499), Titanium Dioxide (CI 77891).
| SUPER BB ALL IN 1 BEAUTY BALM CREAM
titanium dioxide, zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Markwins Beauty Brands (078890119) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.