Flunisolide by Ingenus Pharmaceuticals, LLC / RiconPharma LLC / Mission Pharmacal Company FLUNISOLIDE solution
Flunisolide by
Drug Labeling and Warnings
Flunisolide by is a Prescription medication manufactured, distributed, or labeled by Ingenus Pharmaceuticals, LLC, RiconPharma LLC, Mission Pharmacal Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Flunisolide Nasal Solution USP, 0.025% is intended for administration as a spray to the nasal mucosa. Flunisolide, the active component of flunisolide nasal solution, is an anti-inflammatory steroid.
Flunisolide is represented by the following structural formula:
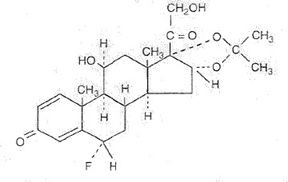
C24H31FO6
Mol. Wt. 434.51Chemical Name: 6α-fluoro-11β, 16α,17,21-tetrahydroxypregna-1,4-di-ene-3,20-dione cyclic 16,17-acetal with acetone (USAN).
Flunisolide is a white to creamy white crystalline powder. It is soluble in acetone, sparingly soluble in chloroform, slightly soluble in methanol, and practically insoluble in water. It has a melting point of about 245°C.
After initial priming (5 to 6 sprays), each spray of the unit delivers a metered droplet spray of 100 mg formulation containing 25 mcg of flunisolide.
The size of the droplets produced by the unit is in excess of 8 microns to facilitate deposition on the nasal mucosa. The contents of one nasal spray bottle delivers 200 sprays.
Each 25 mL flunisolide nasal solution spray bottle contains 6.25 mg (0.25 mg/mL).
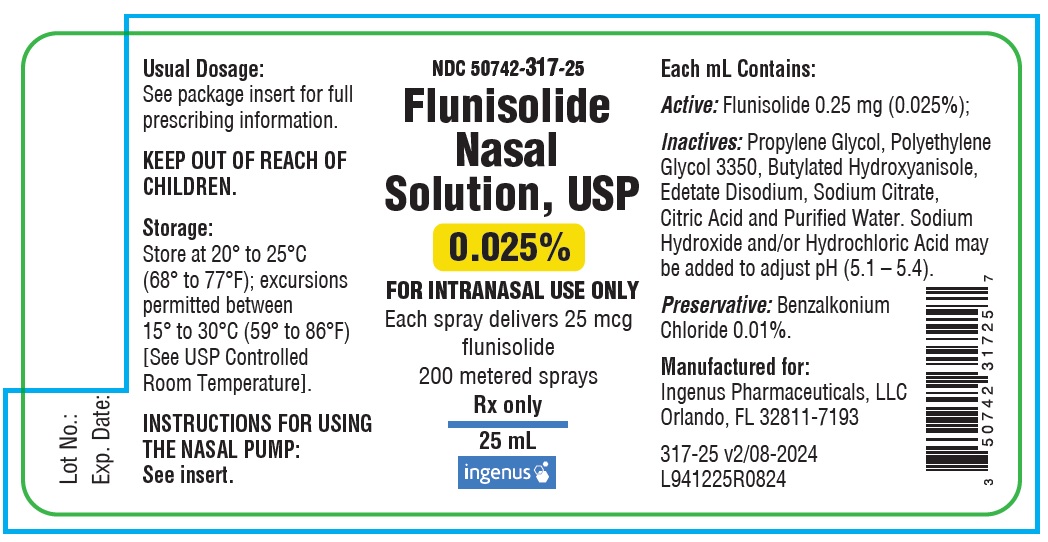
Each mL Contains: ACTIVE: Flunisolide 0.25 mg (0.025%); INACTIVES: Propylene Glycol, Polyethylene Glycol 3350, Butylated Hydroxyanisole, Edetate Disodium, Sodium Citrate, Citric Acid, and Purified Water. Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (5.1 – 5.4). PRESERVATIVE: Benzalkonium Chloride 0.01%.
-
CLINICAL PHARMACOLOGY
Flunisolide has demonstrated potent glucocorticoid and weak mineralocorticoid activity in classical animal test systems. As a glucocorticoid, it is several hundred times more potent than the cortisol standard. Clinical studies with flunisolide have shown therapeutic activity on nasal mucous membranes with minimal evidence of systemic activity at the recommended doses.
A study in approximately 100 patients that compared the recommended dose of flunisolide nasal solution with an oral dose providing equivalent systemic amounts of flunisolide has shown that the clinical effectiveness of flunisolide nasal solution, when used topically as recommended, is due to its direct local effect and not to an indirect effect through systemic absorption.
Following administration of flunisolide to man, approximately half of the administered dose is recovered in the urine and half in the stool: 65 to 70% of the dose recovered in urine is the primary metabolite, which has undergone loss of the 6α fluorine and addition of a 6β hydroxy group. Flunisolide is well absorbed but is rapidly converted by the liver to the much less active primary metabolite and to glucuronate and/or sulfate conjugates. Because of first-pass liver metabolism, only 20% of the flunisolide reaches the systemic circulation when it is given orally whereas 50% of the flunisolide administered intranasally reaches the systemic circulation unmetabolized. The plasma half-life of flunisolide is 1 to 2 hours.
The effects of flunisolide on hypothalamic-pituitary-adrenal (HPA) axis function have been studied in adult volunteers. Flunisolide was administered intranasally as a spray in total doses over 7 times the recommended dose (2200 mcg, equivalent to 88 sprays/day) in 2 subjects for 4 days, about 3 times the recommended dose (800 mcg, equivalent to 32 sprays/day) in 4 subjects for 4 days, and over twice the recommended dose (700 mcg, equivalent to 28 sprays/day) in 6 subjects for 10 days. Early morning plasma cortisol concentrations and 24-hour urinary 17-ketogenic steroids were measured daily. There was evidence of decreased endogenous cortisol production at all three doses.
In controlled studies, flunisolide nasal solution was found to be effective in reducing symptoms of stuffy nose, runny nose and sneezing in most patients. These controlled clinical studies have been conducted in 488 adult patients at doses ranging from 8 to 16 sprays (200-400 mcg) per day and 127 pediatric patients at doses ranging from 6 to 8 sprays (150 to 200 mcg) per day for periods as long as 3 months. In 170 patients who had cortisol levels evaluated at baseline and after 3 months or more of flunisolide treatment, there was no unequivocal flunisolide-related depression of plasma cortisol levels.
Clinical studies have shown that improvement is usually apparent within a few days after starting flunisolide nasal solution.
The mechanisms responsible for the anti-inflammatory action of corticosteroids and for the activity of the aerosolized drug on the nasal mucosa are unknown.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
The replacement of a systemic corticosteroid with a topical corticoid can be accompanied by signs of adrenal insufficiency, and in addition some patients may experience symptoms of withdrawal, e.g., joint and/or muscular pain, lassitude and/or depression. Patients previously treated for prolonged periods with systemic corticosteroids and transferred to flunisolide should be carefully monitored to avoid acute adrenal insufficiency in response to stress.
When transferred to flunisolide, careful attention must be given to patients previously treated for prolonged periods with systemic corticosteroids. This is particularly important in those patients who have associated asthma or other clinical conditions, where too rapid a decrease in systemic corticosteroids may cause a severe exacerbation of their symptoms.
The use of flunisolide with alternate-day prednisone systemic treatment could increase the likelihood of HPA suppression compared to a therapeutic dose of either one alone. Therefore, flunisolide treatment should be used with caution in patients already on alternate-day prednisone regimens for any disease.
Persons who are on drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in non-immune pediatric patients or adults on corticosteroids. In such pediatric patients or adults who have not had these diseases, particular care should be taken to avoid exposure. How the dose, route and duration of corticosteroid administration affects the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a nonimmune patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package insert for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
-
PRECAUTIONS
General
Intranasal corticosteroids may also cause a reduction in growth velocity when administered to pediatric patients (see PRECAUTIONS, Pediatric Use).
Symptomatic relief may not occur in some patients for as long as 2 weeks. Although systemic effects are minimal at recommended doses, flunisolide should not be continued beyond 3 weeks in the absence of significant symptomatic improvement. In clinical studies with flunisolide administered intranasally, the development of localized infections of the nose and pharynx with Candida albicans has occurred only rarely. When such an infection develops, it may require treatment with appropriate local therapy or discontinuance of treatment with flunisolide.
Flunisolide is absorbed into the circulation. Use of excessive doses of flunisolide may suppress HPA function. Flunisolide should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract or in untreated fungal, bacterial or systemic viral infections or ocular herpes simplex.
Because of the inhibitory effect of corticosteroids on wound healing, in patients who have experienced recent nasal septal ulcers, recurrent epistaxis, nasal surgery or trauma, a nasal corticosteroid should be used with caution until healing has occurred.
Although systemic effects have been minimal with recommended doses, this potential increases with excessive dosages. Therefore, larger than recommended doses should be avoided.
Information for Patients
Patients should use flunisolide at regular intervals since its effectiveness depends on its regular use. The patient should take the medication as directed. It is not acutely effective and the prescribed dosage should not be increased. Instead, nasal vasoconstrictors or oral antihistamines may be needed until the effects of flunisolide are fully manifested. One to two weeks may pass before full relief is obtained. The patient should contact the physician if symptoms do not improve, or if the condition worsens, or if sneezing or nasal irritation occurs.
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
For the proper use of this unit and to attain maximum improvement, the patient should read and follow the accompanying Patient Instructions carefully.
Patients should be advised to clear their nasal passages of secretions prior to use.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In mice, flunisolide at an oral dose of 500 mcg/kg/day (approximately 6 times the maximum recommended daily intranasal dose in adults and children on a mg/m2 basis) for 21 months was negative for carcinogenic effects. In rats, administration of flunisolide at an oral dose of 2.5 mcg/kg/day (less than the maximum recommended daily intranasal dose in adults and children on a mg/m2 basis) for 24 months resulted in an increased incidence of mammary gland adenoma and islet cell adenoma of the pancreas in females. There were no significant increases in the incidence of any tumor type in rats at an oral dose of 1.0 mcg/kg (less than the maximum recommended daily intranasal dose in adults and children on a mg/m2 basis).
Flunisolide showed no mutagenic activity in in vitro test systems including the Ames Assay and the Rec-Assay, and no clastogenic activity in either the in vitro chromosomal aberration assay in Chinese hamster lung fibroblast cells or the in vivo mouse bone marrow chromosomal aberration assay.
Flunisolide, at an oral dose of 200 mcg/kg/day (approximately 4 times the maximum recommended daily intranasal dose in adults on a mg/m2 basis) produced impaired fertility in female rats, but was devoid of such effect at oral doses less than or equal to 40 mcg/kg/day (approximately equal to the maximum recommended daily intranasal dose in adults on a mg/m2 basis).
Pregnancy
As with other corticosteroids, flunisolide has been shown to be teratogenic and fetotoxic in rabbits and rats at oral doses of 40 and 200 mcg/kg/day, respectively (approximately 2 and 4 times, respectively, the maximum recommended daily intranasal dose in adults on a mg/m2 basis). There are no adequate and well-controlled studies in pregnant women. Flunisolide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because other corticosteroids are excreted in human milk, caution should be exercised when flunisolide is administered to nursing women.
Pediatric Use
Flunisolide nasal solution is not recommended for use in pediatric patients less than 6 years of age as safety and efficacy have not been assessed in this age group. Controlled clinical studies have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for “catch up” growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving intranasal corticosteroids, including flunisolide nasal solution, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of safe and effective noncorticosteroid treatment alternatives. To minimize the systemic effects of intranasal corticosteroids, including flunisolide nasal solution, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
Geriatric Use
Clinical studies of flunisolide nasal solution did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose reduction for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting a greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Adverse reactions reported in controlled clinical trials and long-term open studies in 595 patients treated with flunisolide nasal solution are described below. Of these patients, 409 were treated for 3 months or longer, 323 for 6 months or longer, 259 for 1 year or longer, and 91 for 2 years or longer.
In general, side effects elicited in the clinical studies have been primarily associated with the nasal mucous membranes. The most frequent complaints were those of mild transient nasal burning and stinging, which were reported in approximately 45% of the patients treated with flunisolide nasal solution in placebo-controlled and long-term studies. These complaints do not usually interfere with treatment; in only 3% of patients was it necessary to decrease dosage or stop treatment because of these symptoms. Approximately the same incidence of mild transient nasal burning and stinging was reported in patients on placebo as was reported in patients treated with flunisolide nasal solution in controlled studies, implying that these complaints may be related to the vehicle or the delivery system. The incidence of complaints of nasal burning and stinging decreased with increasing duration of treatment.
Other side effects reported at a frequency of 5% or less were: nasal congestion, sneezing, epistaxis and/or bloody mucous, nasal irritation, watery eyes, sore throat, nausea and/or vomiting, and headaches. As with other nasally inhaled corticosteroids, nasal septal perforations have been reported in rare instances with the use of flunisolide nasal solutions. Temporary or permanent loss of the sense of smell and taste have also been reported with the use of flunisolide nasal solutions.
Systemic corticosteroid side effects were not reported during the controlled clinical trials. If recommended doses are exceeded, or if individuals are particularly sensitive, symptoms of hypercorticism, i.e., Cushing’s syndrome, could occur.
Cases of growth suppression have been reported for intranasal corticosteroids (including flunisolide nasal solution) (see PRECAUTIONS, Pediatric Use).
To report SUSPECTED ADVERSE REACTIONS, contact Ingenus Pharmaceuticals, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Adults: The recommended starting dose of flunisolide nasal solution is 2 sprays (50 mcg) in each nostril 2 times a day (total dose 200 mcg/day). If needed, this dose may be increased to 2 sprays in each nostril 3 times a day (total dose 300 mcg/day).
Pediatric Patients 6 to 14 years: The recommended starting dose of flunisolide nasal solution is 1 spray (25 mcg) in each nostril 3 times a day or 2 sprays (50 mcg) in each nostril 2 times a day (total dose 150 to 200 mcg/day). Flunisolide nasal solution is not recommended for use in pediatric patients less than 6 years of age as safety and efficacy studies, including possible adverse effects on growth, have not been conducted.
Maximum total daily doses should not exceed 8 sprays in each nostril for adults (total dose 400 mcg/day) and 4 sprays in each nostril for pediatric patients under 14 years of age (total dose 200 mcg/day). Since there is no evidence that exceeding the maximum recommended dosage is more effective and increased systemic absorption would occur, higher doses should be avoided.
After the desired clinical effect is obtained, the maintenance dose should be reduced to the smallest amount necessary to control the symptoms. Approximately 15% of patients with perennial rhinitis may be maintained on as little as 1 spray in each nostril per day.
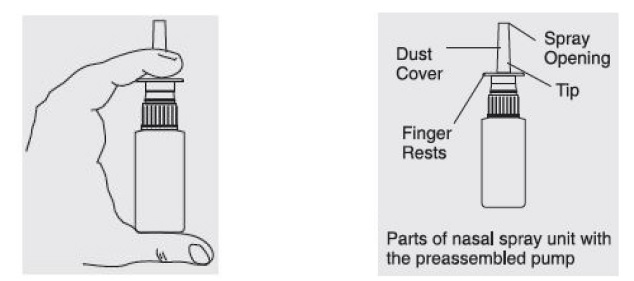
For priming and repriming the nasal spray unit after storage: The patient should remove the dust cover. Put two fingers on “shoulders” of pump unit, and place thumb on bottom of bottle. Push bottle with thumb FIRMLY and QUICKLY 5-6 times or until fine spray appears. Now your pump is primed. The patient must prime the pump unit again if it has not been used for 5 days or more, or if it has been disassembled for cleaning.
- DIRECTIONS FOR USE
- WARNING
- HOW SUPPLIED
- Storage
-
PATIENT INSTRUCTIONS
PHARMACIST - DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
PATIENT INSTRUCTIONS
Flunisolide Nasal Solution USP, 0.025%
– HOW TO USE YOUR NASAL PUMP UNIT –Your nasal spray unit with the pump requires no assembly.
Just follow the simple instructions below.IMPORTANT INFORMATION ON FLUNISOLIDE NASAL SOLUTION:
- You should use flunisolide nasal solution at regular intervals since its effectiveness depends on its regular use (see below).
- It may take 1-2 weeks before full relief is obtained.
- You should contact your physician if symptoms do not improve, if the condition worsens, or if sneezing, nasal irritation, or bleeding occurs.
- You should contact your physician if you know you have been exposed to chickenpox or measles.
TO PRIME:
- Remove the dust cover. Put two fingers on “shoulders” of pump unit, and place thumb on bottom of bottle. Push bottle with thumb FIRMLY and QUICKLY 5-6 times or until a fine spray appears. Now your pump is primed. You must prime the pump unit again if you have not used it for 5 days or more, or if you have disassembled it for cleaning.
- If the solution is delivered in a stream of liquid, it may fail to provide maximum benefit and cause some discomfort. A fine mist can be produced only by a rapid and firm pumping action.
- Once your pump is primed, it's ready to use.
NOTE: Keep dust cover on the pump unit when not in use.
TO USE:
- Gently blow nose to clear nostrils. If nose is blocked, use medicine your doctor has recommended to open nasal passages.
2. Remove the dust cover. Be sure the pump unit is primed.
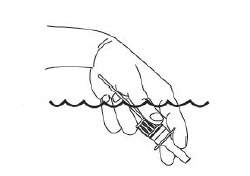
3. Place the spray tip into one nostril (tip should not reach far into nose). Bend head forward so spray will aim toward the back of the nose.
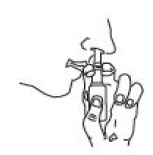
4. Hold pump as shown, resting back of index finger against upper lip.
BE CAREFUL THAT FINGERS DO NOT SLIP OFF THE PUMP AS YOU SPRAY.
5. Point the tip toward the BACK and OUTER SIDE of the nose. Close other nostril with finger. Pump the spray by pushing bottle with thumb FIRMLY and QUICKLY and sniff gently at the same time. Your doctor will tell you whether to pump once or twice in each nostril.

6. After spraying in nostril, remove unit from nose and bend head back for a few seconds to let spray spread over back of nose.
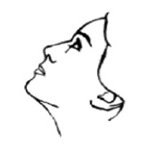
7. Repeat Steps 5 and 6 in other nostril. You may feel brief burning or stinging after using the spray.
8. Keep dust cover on the pump unit when not in use.
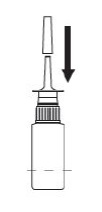
TO CLEAN:
- If spray nozzle becomes clogged, DO NOT ATTEMPT TO CLEAR IT USING A POINTED OBJECT. Remove the pump unit from bottle.
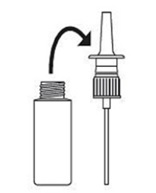
2. Soak only the pump unit in warm water. Squirt several times while holding under water.
3. Make sure the pump unit is dry. Assemble as before with 5 or 6 sprays before use.
Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811-7193
Rev. 08/2024
I9412R0824
-
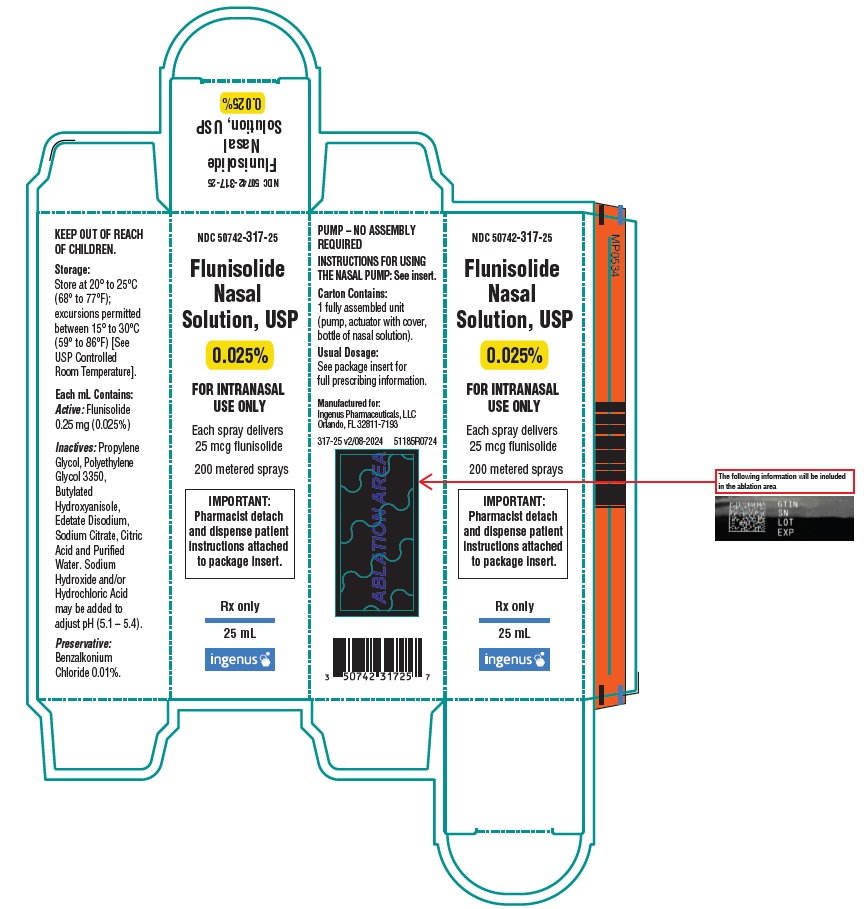
Principal Display Panel
Carton
NDC: 50742-317-25
Flunisolide Nasal Solution, USP
0.025%
FOR INTRANASAL USE ONLY
Each spray delivers 25 mcg flunisolide
200 metered sprays
IMPORTANT: Pharmacist detach and dispense patient instructions attached to package insert.
Rx only
25 mL
Label
NDC: 50742-317-25
Flunisolide Nasal Solution, USP
0.025%
FOR INTRANASAL USE ONLY
Each spray delivers 25 mcg flunisolide
200 metered sprays
Rx only
25 mL
-
INGREDIENTS AND APPEARANCE
FLUNISOLIDE
flunisolide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50742-317 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUNISOLIDE (UNII: QK4DYS664X) (FLUNISOLIDE ANHYDROUS - UNII:78M02AA8KF) FLUNISOLIDE 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 0.1 mg in 1 mL BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50742-317-25 1 in 1 CARTON 06/23/2022 1 25 mL in 1 BOTTLE, PUMP; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207802 06/23/2022 Labeler - Ingenus Pharmaceuticals, LLC (833250017) Registrant - RiconPharma LLC (859035318) Establishment Name Address ID/FEI Business Operations Mission Pharmacal Company 927726893 MANUFACTURE(50742-317)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.