ZO® Skin Health Daily Sheer Broad-Spectrum Sunscreen SPF 45 National Drug Code 42851-094-45 Formula No. ZO-004-171 Bentley (Homosalate Reformulation)
ZO Skin Health Daily Sheer Broad-Spectrum Sunscreen SPF 45 by
Drug Labeling and Warnings
ZO Skin Health Daily Sheer Broad-Spectrum Sunscreen SPF 45 by is a Otc medication manufactured, distributed, or labeled by ZO Skin Health, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZO SKIN HEALTH DAILY SHEER BROAD-SPECTRUM SUNSCREEN SPF 45- avobenzone, homosalate, octisalate, octocrylene lotion
ZO Skin Health, Inc.
----------
ZO® Skin Health Daily Sheer Broad-Spectrum Sunscreen SPF 45
National Drug Code 42851-094-45
Formula No. ZO-004-171 Bentley (Homosalate Reformulation)
Drug Facts
Active Ingredients
| Active Ingredients | Purpose |
| Avobenzone 3.0% ............. | Sunscreen |
| Homosalate 6.8% ............. | Sunscreen |
| Octisalate 5.0%................ | Sunscreen |
| Octocrylene 10.0% ........... | Sunscreen |
Uses
■ helps prevent sunburn
■ higher SPF gives more sunburn protection
■ provides high protection against sunburn
■ retains SPF after 40 minutes of perspiring
■ if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
■ Apply liberally 15 minutes before sun exposure
■ Reapply: ■ at least every 2 hours ■ after 40 minutes of swimming or sweating
■ Children under 6 months of age: Ask a doctor.
■ Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF value of 15 or higher and other sun protection measures including:
■ limit time in the sun, especially from 10 a.m.-2 p.m.
■ wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients
Water, Butyloctyl Salicylate, Silica, Dimethicone, Cetearyl Alcohol, Diethylhexyl 2,6-Naphthalate, Triacontanyl PVP, Argania Spinosa Kernel Oil, Glyceryl Stearate, PEG-100 Stearate, Tocopheryl Acetate, Isododecane, Glycerin, Bisabolol, Propanediol, Steareth-21, Helianthus Annuus (Sunflower) Seed Oil, Rosmarinus Officinalis (Rosemary) Leaf Extract, Tocopherol, Ascorbic Acid, Retinyl Palmitate, Dipotassium Glycyrrhizate, Squalane, Beta-Glucan, Melanin, Cetearyl Glucoside, Steareth-2, Polysorbate 60, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Acrylates Copolymer, Polymethyl Methacrylate, Disodium EDTA, Ethyl Ferulate, Sodium C14-16 Olefin Sulfonate, Sodium Hydroxide, Phenoxyethanol, Ethylhexylglycerin, Pentylene Glycol, 1,2-Hexanediol, Fragrance, Iron Oxides.
Principal Display Panel
ZO® Skin Health
by Zein Obagi, MD
NDC: 42851-094-45
DAILY SHEER BROAD-SPECTRUM SUNSCREEN SPF 45 WATER RESISTANT
(40 Minutes)
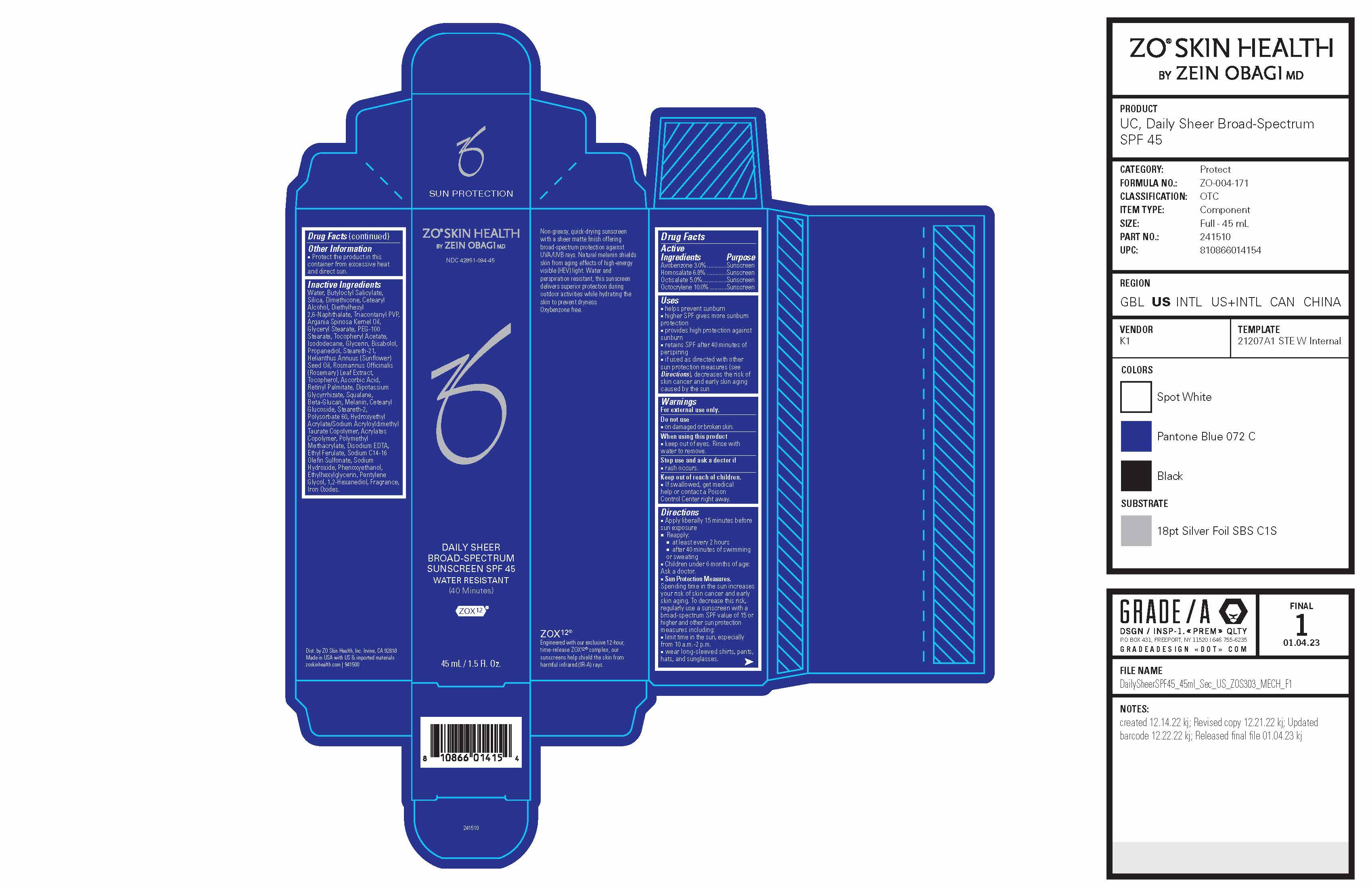
ZOX12®
Engineered with our exclusive 12-hour, time-release ZOX12® complex, our sunscreens help shield the skin from harmful infrared (IR-A) rays.
Non-greasy, quick-drying sunscreen with a sheer matte finish offering broad-spectrum protection against UVA/UVB rays. Natural melanin shields skin from aging effects of high-energy visible (HEV) light. Water and perspiration resistant, this sunscreen delivers superior protection during outdoor activities while hydrating the skin to prevent dryness.
Oxybenzone free.
Dist. by ZO Skin Health, Inc. Irvine, CA 92618 Made in USA with US & imported materials
| ZO SKIN HEALTH DAILY SHEER BROAD-SPECTRUM SUNSCREEN SPF 45
avobenzone, homosalate, octisalate, octocrylene lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ZO Skin Health, Inc. (826468527) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.