PROLEUKIN- aldesleukin injection, powder, lyophilized, for solution
PROLEUKIN by
Drug Labeling and Warnings
PROLEUKIN by is a Prescription medication manufactured, distributed, or labeled by Iovance Biotherapeutics, Inc, Boehringer Ingelheim Pharma GmbH and Co. KG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PROLEUKIN ®safely and effectively. See full prescribing information for PROLEUKIN.
PROLEUKIN (aldesleukin) for injection, for intravenous use
Initial U.S. Approval: 1992WARNING: CAPILLARY LEAK SYNDROME (CLS), NEUROLOGIC TOXICITY, AND SERIOUS INFECTIONS
See full prescribing information for complete boxed warning.
- Capillary Leak Syndrome (CLS) including life-threatening or fatal reactions, has occurred in patients treated with Proleukin. Administer Proleukin in a hospital setting with an intensive care unit. Withhold or discontinue Proleukin as recommended. ( 2.4, 4, 5.1)
- Neurologic toxicities, which may be life-threatening or result in coma or permanent neurological deficits, have occurred in patients treated with Proleukin. Withhold or discontinue Proleukin as recommended. ( 2.4, 5.2)
- Serious infections including sepsis and bacterial endocarditis have occurred in patients treated with Proleukin. Treat preexisting bacterial infections prior to initiating Proleukin and withhold Proleukin as recommended. ( 2.4, 5.3)
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Administer Proleukin in an inpatient hospital setting with an intensive care facility.
- Evaluate cardiovascular, pulmonary, neurologic and renal function before beginning Proleukin. ( 2.1)
- See Full Prescribing Information for Premedication and Supportive medications. ( 2.3)
- Recommended dosage: 600,000 IU/kg (0.037 mg/kg) every 8 hours by a 15-minute intravenous infusion for a maximum of 14 doses. Following 9 days of rest, repeat the schedule for a maximum of another 14 doses (total of 28 doses per course, as tolerated). ( 2.2)
- Evaluate patients approximately 4 weeks after completion of a course of therapy and again immediately prior to the start of the next course. ( 2.2)
- Dose modification for toxicity should be accomplished by withholding or interrupting a dose. ( 2.4)
- Follow reconstitution and dilution procedures. ( 2.5)
DOSAGE FORMS AND STRENGTHS
For Injection: 22 million International Units (1.3 mg) lyophilized powder in a single-dose vial. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Renal Toxicity:Monitor renal function at baseline and throughout treatment. Withhold Proleukin or permanently discontinue, based on severity. ( 2.4, 5.4)
- Immune-mediated Adverse Reactions:Exacerbation of pre-existing autoimmune disease or initial presentation of autoimmune and inflammatory disorders can occur in any system or tissue. Proleukin may increase the risk of allograft rejection in transplant patients. Monitor patients and treat as indicated. ( 5.5)
- Severe Hypersensitivity Reaction:Permanently discontinue Proleukin for severe hypersensitivity reactions. ( 4, 5.9)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of potential risk to a fetus and to use effective contraception. ( 5.6, 8.1, 8.3)
ADVERSE REACTIONS
Most common (≥ 30%) adverse reactions including laboratory abnormalities are hypotension, hyperbilirubinemia, dyspnea, rash, diarrhea, oliguria, chills, vomiting, thrombocytopenia, nausea, confusional state and increased creatinine. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Iovance Biotherapeutics Manufacturing LLC. at 1 844-845-IOVA or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CAPILLARY LEAK SYNDROME (CLS), NEUROLOGIC TOXICITIES and SERIOUS INFECTIONS
1 INDICATIONS AND USAGE
1.1 Metastatic Renal Cell Carcinoma
1.2 Metastatic Melanoma
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluation and Testing Before Initiating Proleukin
2.2 Recommended Dosage
2.3 Premedication and Supportive Medications
2.4 Dosage Modifications for Adverse Reactions
2.5 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Capillary Leak Syndrome
5.2 Neurologic Toxicity
5.3 Serious Infections Including Sepsis
5.4 Renal Toxicity
5.5 Immune-mediated Adverse Reactions
5.6 Embryo-Fetal Toxicity
5.7 Serious Manifestations of Eosinophilia
5.8 Delayed Adverse Reactions to Iodinated Contrast Media
5.9 Severe Hypersensitivity Reactions
5.10 Infusion-Related Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Proleukin
7.2 Effect of Proleukin on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Metastatic Renal Cell Cancer
14.2 Metastatic Melanoma
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CAPILLARY LEAK SYNDROME (CLS), NEUROLOGIC TOXICITIES and SERIOUS INFECTIONS
- Capillary leak syndrome (CLS), including life threatening or fatal reactions, has occurred in patients treated with Proleukin. Do not administer Proleukin to patients with significant cardiac, pulmonary, renal, and hepatic impairment. Administer Proleukin in a hospital setting with an intensive care facility. Withhold or discontinue Proleukin as recommended [see Dosage and Administration (2.4), Contraindications (4), Warnings and Precautions (5.1)] .
- Neurologic toxicities, which may be life-threatening or result in coma or permanent neurological deficits, have occurred in patients treated with Proleukin. Withhold or discontinue Proleukin as recommended [see Dosage and Administration (2.4), Warnings and Precautions (5.2)] .
- Serious Infections including sepsis and bacterial endocarditis have occurred in patients treated with Proleukin. Treat pre-existing bacterial infections prior to initiation of Proleukin therapy and withhold Proleukin as recommended [see Dosage and Administration (2.4), Warnings and Precautions (5.3)] .
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluation and Testing Before Initiating Proleukin
Conduct baseline hematologic, chemistry, renal and hepatic function tests. Additionally, evaluate cardiac ejection fraction, coronary artery disease as appropriate, pulmonary function with PFTs, and evaluate for renal, hepatic, and CNS impairment prior to initiating treatment with Proleukin [see Contraindications (4), Warnings and Precautions (5.1, 5.2)].
Verify pregnancy status of females of reproductive potential prior to initiating Proleukin [see Warnings and Precautions (5.6), Use in Specific Populations (8.1, 8.3)].
2.2 Recommended Dosage
Administer Proleukin in an inpatient hospital setting. An intensive care facility with specialists skilled in cardiopulmonary or intensive care medicine must be available [see Warnings and Precautions (5.1)] .
The recommended dosage of Proleukin for metastatic renal cell carcinoma and metastatic melanoma is described in Table 1.
Administer Proleukin as an intravenous infusion after dilution [see Dosage and Administration (2.5)] .
Administer pre-infusion medications and supportive treatment, as appropriate, prior to and during each infusion [see Dosage and Administration (2.3)]. Discontinue Proleukin for unacceptable toxicity [see Dosage and Administration (2.4)].
Table 1: Recommended Dosage of Proleukin - * A maximum of 28 doses (2 cycles) per treatment course
Each course of therapy consists of the following: Cycle 1 Days 1-5 600,000 IU/kg (0.037 mg/kg) every 8 hours; maximum of 14 doses * Rest period Days 6-14 Cycle 2 Days 15-19 600,000 IU/kg (0.037 mg/kg) every 8 hours; maximum of 14 doses * Evaluate patients for response approximately 4 weeks after completion of a course of therapy and again immediately prior to the scheduled start of the next treatment course.
Additional courses of treatment may be administered to patients if there is a treatment response following the last course, and the patient did not experience any adverse reactions in previous course(s) that led to permanent discontinuation [see Dosage and Administration (2.4)].
Separate each treatment course by a rest period of at least 7 weeks from the date of hospital discharge.
2.3 Premedication and Supportive Medications
Premedicate patients with an antipyretic immediately prior to beginning Proleukin. Continue antipyretics during treatment as needed for fever [see Warnings and Precautions (5.1, 5.10)].
Administer prophylactic antibiotics per institutional guidelines prior to beginning Proleukin and throughout the treatment course for patients with indwelling central catheters [see Warnings and Precautions (5.3)].
Administer prophylactic medication for gastrointestinal irritation and bleeding during each Proleukin treatment course [see Adverse Reactions (6.1)].
Additional medications may be needed if patients experience hypotension, dyspnea, rigors, nausea, diarrhea, pruritis, or dermatitis [see Warnings and Precautions (5.1, 5.8, 5.9)].
2.4 Dosage Modifications for Adverse Reactions
No dose reduction for Proleukin is recommended for adverse reactions. In general, withhold or interrupt a dose or permanently discontinue Proleukin based on the severity of the adverse reaction as described in Table 2.
Table 2: Recommended Dosage Modifications for Adverse Reactions Adverse Reaction Severity Dosage Modification Cardiovascular [see Warnings and Precautions (5.1)] - Atrial fibrillation,
- Supraventricular tachycardia, or
- Bradycardia that requires treatment or is recurrent or persistent
- Decrease in systolic blood pressure to <90 mmHg
Withhold until patient is asymptomatic with full recovery to normal sinus rhythm - Sustained ventricular tachycardia (≥5 beats)
- Cardiac rhythm disturbances not controlled or unresponsive to management
- ECG changes consistent with ischemia or myocardial infarction or angina/chest pain
- Cardiac tamponade
Permanently discontinue Respiratory [see Warnings and Precautions (5.1)] - O2 saturation <90%
Withhold until O2 saturation is >90% - Intubation for >72 hours
Permanently discontinue Neurologic [see Warnings and Precautions (5.2)] - Mental status changes, including moderate confusion or agitation
Withhold until completely resolved - Coma or toxic psychosis lasting >48 hours
- Repetitive or difficult to control seizures
Permanently discontinue Gastrointestinal [see Warnings and Precautions (5.1)] Fecal immunochemical test (FIT) or fecal occult blood test (FOBT) positive Withhold until FIT or FOBT negative Bowel ischemia/perforation or GI bleeding requiring surgery Permanently discontinue Hepatic [see Warnings and Precautions (5.1)] Signs of hepatic toxicity including liver pain or ≥ Grade 3 AST or ALT elevation Withhold all further treatment for that course. Initiate a new course of treatment no sooner than 7 weeks after signs of hepatic toxicity have resolved and hospital discharge Hepatic failure Permanently discontinue Dermatologic [see Warnings and Precautions (5.5, 5.7)] Bullous dermatitis or marked worsening of pre-existing skin condition Withhold until all signs of bullous dermatitis have resolved Infectious [see Warnings and Precautions (5.3)] Sepsis syndrome, patient is clinically unstable Withhold until sepsis syndrome has resolved, patient is clinically stable, infection is under treatment Renal [see Warnings and Precautions (5.1, 5.4)] Serum creatinine >4.5 mg/dL or a serum creatinine of ≥4 mg/dL in the presence of severe volume overload, acidosis, or hyperkalemia Withhold until serum creatinine levels return to normal (<1.5 mg/dL) or baseline and fluid and electrolyte status are stable Persistent oliguria, urine output of <10 mL/hr for 16-24 hours with rising SCr Withhold until urine output >10 mL/hour with a decrease of serum creatinine >1.5 mg/dL or normalization of serum creatinine Renal failure requiring dialysis >72 hours Permanently discontinue 2.5 Preparation and Administration
Preparation
Reconstitute Proleukin using Sterile Water for Injection, USP. Do not reconstitute or dilute Proleukin with Bacteriostatic Water for Injection, or 0.9% Sodium Chloride Injection.
- Add 1.2 mL of Sterile Water for Injection, USP, by injecting the water along the walls of the vial and not directly on the lyophilized powder. The resulting concentration is 18 million IU (1.1 mg)/mL of Proleukin.
- The prepared solution is a clear, colorless to slightly yellow liquid.
- Slowly swirl the vial; do not shake.
- Withdraw the required dose of Proleukin and discard the vial with any unused portion.
- Use polyvinyl chloride bags for dilution of Proleukin and dilute using 5% Dextrose Injection to a concentration between 0.03 mg/mL and 0.07 mg/mL based on the required dose as follows:
Table 3: Recommended Proleukin Dilution Dose 5% Dextrose Volume ≤25.4 million IU (≤1.5 mg) 25 mL >25.4 million IU-60 million IU (>1.5 mg-3.5 mg) 50 mL >60 million IU (>3.5 mg) 100 mL Storage of Diluted Proleukin Infusion Solution
- Store under refrigeration at 2° to 8°C (36° to 46°F) for no more than 48 hours from the time of preparation to the end of the infusion.
- Protect from light.
- Do not freeze.
- Allow the diluted solution to come to room temperature prior to administration.
Administration
- Do not use in-line filters when administering Proleukin.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not co-administer Proleukin with other drugs through the same intravenous line.
- Administer by intravenous infusion over 15 minutes.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Severe Hypersensitivity Reactions
Proleukin is contraindicated in patients with a known history of severe hypersensitivity to aldesleukin or any component of the Proleukin formulation [see Adverse Reactions (6.2)].
- Organ Allografts
Proleukin is contraindicated in patients with organ allografts [see Warnings and Precautions (5.5)] .
- Significant Organ Impairment
Proleukin is contraindicated in patients with significant cardiac (including those with an abnormal cardiac ejection fraction, impaired wall motion, or significant coronary artery disease), pulmonary (including those with an FEV1 ≤ 2 liters or < 75% predicted for height and age), renal, hepatic, or CNS impairment [see Warnings and Precautions (5.1, 5.2, 5.4)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Capillary Leak Syndrome
Severe and life-threatening capillary leak syndrome (CLS) characterized by hypotension, dyspnea, edema, and hypoalbuminemia can occur with Proleukin, and can result in end organ toxicity including cardiac, respiratory, renal, hepatic toxicity, or death. Do not administer Proleukin to patients with significant cardiac, pulmonary, renal, or hepatic impairment. Avoid concomitant use of Proleukin with other products known to cause hypotension including antihypertensive drugs, those that cause renal toxicity, or hepatotoxicity.
CLS may begin immediately after Proleukin treatment is initiated. Monitor for signs and symptoms of CLS including assessments of vital signs, weight, fluid intake, albumin levels and urine output.
Withhold or discontinue Proleukin for failure to maintain organ perfusion as demonstrated by altered mental status, reduced urine output, oxygen saturation <90%, a fall in the systolic blood pressure below 90 mm Hg, or onset of cardiac arrhythmias. Initiate standard management for CLS, which may include intensive care [see Dosage and Administration (2.4), Use in Specific Populations (8.1)] .
5.2 Neurologic Toxicity
Proleukin can cause neurologic toxicities including mental status changes, speech difficulties, cortical blindness, limb or gait ataxia, hallucinations, agitation, obtundation, demyelinating polyneuropathy, and coma. Alterations in mental status may progress for several days before recovery begins. Permanent neurologic deficits have occurred. Radiological findings included multiple and, less commonly, single cortical lesions on MRI and evidence of demyelination. One case of possible cerebral vasculitis has been reported.
Monitor patients for signs and symptoms of neurological toxicity during Proleukin treatment. Withhold Proleukin in patients developing moderate to severe lethargy or somnolence; continued administration may result in coma. Permanently discontinue Proleukin for coma or toxic psychosis lasting >48 hours or for repetitive or difficult to control seizures [see Dosage and Administration (2.4)].
Evaluate and treat CNS metastases prior to initiation of Proleukin. If possible, avoid concomitant use of Proleukin with other product(s) with a known potential to cause neurotoxicity, and avoid Proleukin in patients with seizure disorders or abnormal intracranial imaging [see Contraindications (4), Adverse Reactions (6.1, 6.2)] . Concomitant use of Proleukin with other products that cause neurotoxicity may result in a greater risk of severe neurotoxicity.
5.3 Serious Infections Including Sepsis
Proleukin can cause impaired neutrophil function (reduced chemotaxis) and an increased risk of disseminated infection, including sepsis and bacterial endocarditis. Treat pre-existing bacterial infections prior to initiating Proleukin. Consider antibiotic prophylaxis in patients with indwelling central lines. Monitor patients for the development of signs and symptoms of infection during treatment and withhold Proleukin based on severity [see Dosage and Administration (2.4)].
5.4 Renal Toxicity
Serious renal toxicity, including oliguria and renal failure can occur with Proleukin [see Adverse Reactions (6.1, 6.2)] . Pre-existing renal impairment or coadministration of Proleukin with other products known to cause renal toxicity may increase this risk. If possible, avoid concomitant use of Proleukin with other product(s) with a known potential to cause renal toxicity. Serum creatinine should be ≤1.5 mg/dL before beginning Proleukin. Monitor serum creatinine at baseline and daily throughout each course of therapy. Withhold Proleukin, or permanently discontinue, based on severity [see Dosage and Administration (2.4)] .
5.5 Immune-mediated Adverse Reactions
Exacerbation of pre-existing autoimmune disease or initial presentation of autoimmune and inflammatory disorders has been reported following treatment with Proleukin. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. These have included exacerbation of Crohn's disease, colitis, scleroderma, thyroiditis, inflammatory arthritis, diabetes mellitus, oculo-bulbar myasthenia gravis, crescentic IgA glomerulonephritis, cholecystitis, cerebral vasculitis, Stevens-Johnson syndrome, bullous pemphigoid, myocarditis, myositis, and neuritis including optic neuritis resulting in blindness [see Adverse Reactions (6.2)] .
Proleukin may increase the risk of allograft rejection in transplant patients [see Contraindications (4)] .
Hypothyroidism, sometimes preceded by hyperthyroidism, has been reported following Proleukin treatment. Evaluate thyroid function at baseline and periodically during treatment and initiate thyroid replacement therapy as clinically indicated.
Hyperglycemia and/or diabetes mellitus has been reported during Proleukin therapy. Monitor patients for hyperglycemia and initiate treatment with insulin as clinically indicated.
5.6 Embryo-Fetal Toxicity
Based on findings in an animal study and its mechanism of action, Proleukin may cause fetal harm or loss of pregnancy when administered to a pregnant woman. In pregnant rats, aldesleukin has been shown to have embryolethal effects at doses 27 times and maternal toxicities at doses 2.1 times the human exposure at the recommended clinical dose. Advise pregnant women of the potential risk to a fetus. Advise female patients of reproductive potential to use effective contraception during treatment with Proleukin [see Use in Specific Populations (8.1, 8.3)].
5.7 Serious Manifestations of Eosinophilia
Serious manifestations of eosinophilia involving eosinophilic infiltration of cardiac and pulmonary tissues can occur following Proleukin.
5.8 Delayed Adverse Reactions to Iodinated Contrast Media
A review of the literature revealed that 12.6% (range 11-28%) of 501 patients treated with various interleukin-2-containing regimens who were subsequently administered radiographic iodinated contrast media experienced acute, atypical adverse reactions. The onset of symptoms usually occurred within hours (most commonly 1 to 4 hours) following the administration of contrast media. These reactions include fever, chills, nausea, vomiting, pruritus, rash, diarrhea, hypotension, edema, and oliguria. These reactions may resemble the immediate side effects caused by interleukin-2 administration. Most events were reported to occur when contrast media was given within 4 weeks after the last dose of interleukin-2. These events were also reported to occur when contrast media was given several months after interleukin-2 treatment [see Adverse Reactions (6.2)] .
5.9 Severe Hypersensitivity Reactions
Proleukin can cause severe hypersensitivity reactions, including anaphylactic reactions. Permanently discontinue Proleukin in patients who experience a severe hypersensitivity reaction [see Contraindications (4), Adverse Reactions (6.2)].
5.10 Infusion-Related Reactions
Proleukin can cause fevers, chills, or rigors. Premedicate patients with an antipyretic prior to beginning Proleukin and continue during treatment as needed for fever [see Adverse Reactions (6.2)and Dosage and Administration (2.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Capillary Leak Syndrome [see Warnings and Precautions (5.1)].
- Neurotoxicity [see Warnings and Precautions (5.2)].
- Serious Infections Including Sepsis [see Warnings and Precautions (5.3)].
- Renal Toxicity [see Warnings and Precautions (5.4)].
- Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.5)].
- Serious Manifestations of Eosinophilia [see Warnings and Precautions (5.7)].
- Severe Hypersensitivity Reactions [see Warnings and Precautions (5.9)].
- Infusion-Related Reactions [see Warnings and Precautions (5.10)] .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of Proleukin was evaluated in a series of single and multicenter, controlled studies enrolling a total of 525 patients with metastatic renal cell carcinoma (mRCC studies) or metastatic melanoma (metastatic melanoma studies) [see Clinical Studies (14.1, 14.2)] .
In patients who received Proleukin in these studies, fatal adverse reactions occurred in 4% (11/255) of the patients with metastatic RCC, and 2% (6/270) of the patients with metastatic melanoma.
In these studies, >90% of patients had doses withheld for adverse reactions [see Dosage and Administration (2.4)].
The most common (≥30%) adverse reactions were hypotension, hyperbilirubinemia, dyspnea, rash, diarrhea, oliguria, chills, vomiting, thrombocytopenia, nausea, confusional state, and increased creatinine.
Table 4 summarizes adverse reactions that occurred in these studies.
Table 4: Adverse Reactions (≥10% all grades or ≥1% Grade 4) in Patients with Metastatic Renal Cell Carcinoma or Metastatic Melanoma (n=525) receiving Proleukin Adverse Reaction Proleukin
N = 525All Grades (%) Grade 4 (%) - * Cardiovascular disorder: Electrocardiogram abnormal, cardiac failure congestive.
- † Lung disorder: Pulmonary congestion, rales, rhonchi.
- ‡ Respiratory disorder: Acute respiratory distress syndrome, lung infiltration, lung disorder, respiratory failure, endotracheal intubation.
General disorders Chills 52 0 Pyrexia 29 1 Edema peripheral 28 0 Malaise 27 0 Asthenia 23 0 Edema 15 0 Pain 12 0 Cardiac disorders Hypotension 71 3 Blood pressure fluctuation Not documented 1 Tachycardia 23 0 Dilated veins 13 0 Supraventricular tachycardia 12 1 Ventricular tachycardia <10 1 Cardiovascular disorder * 11 0 Myocardial infarction <10 1 Arrhythmia 10 0 Cardiac arrest <10 1 Gastrointestinal disorders Diarrhea 67 2 Vomiting 50 0 Nausea 35 0 Stomatitis 22 <1 Decreased appetite 20 0 Abdominal pain 11 0 Abdominal distention 10 0 Blood and lymphatic system disorders Thrombocytopenia 37 1 Anemia 29 0 Leukopenia 16 0 Disseminated intravascular coagulation <10 1 Infections Infections 13 1 Sepsis <10 1 Hepatobiliary disorders Hyperbilirubinemia 40 2 Aspartate aminotransferase increased 23 1 Metabolic and nutritional disorders Weight increased 16 0 Acidosis 12 1 Hypomagnesemia 12 0 Hypocalcemia 11 <1 Blood alkaline phosphatase increased 10 0 Nervous system disorders Confusional state 34 1 Stupor <10 1 Coma <10 2 Psychotic disorder <10 1 Somnolence 22 0 Anxiety 12 0 Dizziness 11 0 Respiratory, thoracic, and mediastinal disorders Dyspnea 43 1 Lung disorder † 24 0 Respiratory disorder ‡ 11 3 Apnea <10 1 Cough 11 0 Rhinitis 10 0 Skin and subcutaneous tissue disorders Rash 42 0 Pruritis 24 0 Dermatitis exfoliative 18 0 Renal and urinary disorders Oliguria 63 6 Blood creatinine increased 33 1 Anuria <10 5 Acute kidney injury <10 1 Additional life-threatening adverse reactions (Grade 4) were reported by <1% of the 525 patients:
- Cardiac disorders: bradycardia, pericardial effusion, ventricular extrasystoles, myocardial ischemia, arrhythmia supraventricular, coronary artery disease, atrioventricular block second degree, endocarditis
- Eye disorders: mydriasis, pupillary disorder
- Gastrointestinal disorders: intestinal perforation, gastrointestinal hemorrhage, hematemesis, pancreatitis, diarrhea hemorrhagic
- General disorders and administration site conditions: hypothermia
- Infections and infestations: gangrene
- Metabolism and nutrition disorders: hyperuricemia
- Nervous system disorders: syncope, neuropathy peripheral, seizure, generalized tonic-clonic seizure
- Investigations: liver function tests abnormal, blood urea increased
- Psychiatric disorders: agitation, paranoia
- Renal and urinary disorders: renal failure, renal tubular necrosis
- Respiratory, thoracic and mediastinal disorders: respiratory acidosis, asthma, pulmonary edema, hyperventilation, hypoxia, hemoptysis, hypoventilation, pneumothorax
- Vascular disorders: shock, hemorrhage, phlebitis, thrombosis
Other Clinical Trial Experience
The following serious adverse reactions were reported in patients with RCC, melanoma, or other cancers treated with Proleukin-based regimens (n >1800 patients) using dosages other than the recommended dosage:
- Cardiovascular disorders: transient ischemic attacks, pericarditis
- Gastrointestinal disorders: duodenal ulcer; gastrointestinal necrosis, tracheo-esophageal fistula
- Nervous system disorders: meningitis, brain edema
- Renal and urinary disorders: nephritis (allergic)
In the same clinical population, the following fatal events each occurred with a frequency of <1%: hyperthermia malignant; cardiac arrest; myocardial infarction; pulmonary embolism; cerebrovascular accident; intestinal perforation; hepatic failure or renal failure; severe depression leading to suicide; pulmonary edema; respiratory arrest; respiratory failure. Patients with ECOG PS of 1 or higher had a higher treatment-related mortality and serious adverse events.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Proleukin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and lymphatic system disorders: neutropenia, febrile neutropenia, eosinophilia, lymphopenia
- Cardiac disorders: cardiomyopathy, cardiac tamponade
- Endocrine disorders: hyperthyroidism
- Gastrointestinal disorders: gastritis, intestinal obstruction
- General disorders and administration site conditions: injection site necrosis
- Hepatobiliary disorders: hepatitis, hepatosplenomegaly, cholecystitis
- Immune system disorders: anaphylactic reaction, angioedema, urticaria
- Infections and infestations: pneumonia (bacterial, fungal, viral), endocarditis, cellulitis
- Metabolism and nutrition disorders: hyponatremia, hypophosphatemia
- Musculoskeletal and connective tissue disorders: myopathy, rhabdomyolysis
- Nervous system disorders: encephalopathy, extrapyramidal disorder, neuralgia
- Psychiatric disorders: insomnia
- Vascular disorders: hypertension, subdural hemorrhage, subarachnoid hemorrhage, cerebral hemorrhage, retroperitoneal hemorrhage
-
7 DRUG INTERACTIONS
Drug interaction studies with Proleukin have not been conducted. The drug interaction information described below have been observed post-marketing.
7.2 Effect of Proleukin on Other Drugs
Radiographic Iodinated Contrast Media
Monitor for delayed adverse reactions in patients receiving iodinated contrast media following Proleukin. Administration of radiographic iodinated contrast media following administration of interleukin-2 resulted in acute, atypical adverse reactions that resemble the immediate side effects caused by Proleukin in some patients [see Warnings and Precautions (5.8)] .
Effect on Cytochrome P-450 Substrates
For certain CYP substrates, minimal changes in the concentration may lead to serious adverse reactions. Monitor for toxicity or drug concentration changes of such CYP substrates when co-administered with Proleukin.
Aldesleukin causes release of cytokines [see Clinical Pharmacology (12.2)] that may suppress activity of CYP enzymes, resulting in increased exposure of CYP substrates.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in an animal study and its mechanism of action, Proleukin may cause fetal harm or loss of pregnancy when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Data on the use of Proleukin in pregnant women are limited and insufficient to assess the drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes; however, development of capillary leak syndrome during pregnancy can lead to adverse fetal outcomes (see Clinical Considerations) .
Intravenous administration of aldesleukin to pregnant rats during the period of organogenesis resulted in embryo lethality at doses 27 times and maternal toxicities at doses 2.1 times the human exposure at the recommended clinical dose (see Data) . Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%–4% and 15–20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Capillary leak syndrome in women who are exposed to Proleukin during pregnancy may result in maternal hypotension and decreased placental perfusion. Severe or prolonged maternal hypotension and decreased placental perfusion can lead to intrauterine growth restriction, perinatal asphyxia, or fetal/neonatal demise. Monitor fetal and neonatal status in pregnant women who develop capillary leak syndrome associated with Proleukin.
Data
Animal Data
Aldesleukin has been shown to have embryolethal effects in rats when given in doses at 27 to 36 times the human dose (scaled by body weight). Significant maternal toxicities were observed in pregnant rats administered aldesleukin by IV injection at doses 2.1 to 36 times higher than the human dose during critical period of organogenesis.
8.2 Lactation
Risk Summary
There are no data on the presence of aldesleukin in either human or animal milk, the effects on the breastfed child, or the effects on milk production. Maternal cytokines are known to be present in human breast milk. Because of the potential for serious adverse reactions from Proleukin in a breastfed child, such as impaired immune function, advise women not to breastfeed during treatment.
8.3 Females and Males of Reproductive Potential
Based on animal data and mechanism of action, Proleukin may cause embryo-fetal harm [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiating Proleukin [see Use in Specific Populations (8.1)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Aldesleukin is a human interleukin-2 lymphocyte growth factor produced by recombinant DNA technology using a genetically engineered E. colistrain containing an analog of the human interleukin-2 gene. It is a purified protein with a molecular weight of approximately 15,300 Da. This recombinant form differs from native interleukin-2 in the following ways: a) aldesleukin is not glycosylated; b) the molecule has no N-terminal alanine; c) the molecule has serine substituted for cysteine at amino acid position 125. Proleukin exists as biologically active, non-covalently bound microaggregates with an average size of 27 recombinant interleukin-2 molecules; the aggregation state of aldesleukin may differ compared to native interleukin-2.
The manufacturing process for aldesleukin involves fermentation in a defined medium containing tetracycline hydrochloride. The presence of tetracycline hydrochloride is not detectable in the final product.
Proleukin (aldesleukin) for injection is a sterile, preservative-free white to off-white, lyophilized powder, which has a cake-like appearance, supplied in single-dose vials for intravenous administration after reconstitution and dilution. When reconstituted with 1.2 mL Sterile Water for Injection, USP, each mL contains 18 million International Units (1.1 mg) aldesleukin, mannitol (50 mg), sodium dodecyl sulfate (0.19 mg), buffered with disodium hydrogen phosphate dihydrate (1.12 mg) and sodium dihydrogen phosphate dihydrate (0.19 mg) to a pH of 7.5 (range 7.2 to 7.8).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Aldesleukin is an interleukin-2 lymphocyte growth factor. The antitumor activity of aldesleukin has not been fully characterized. In vitrostudies performed on human cell lines show enhancement of lymphocyte mitogenesis and cytotoxicity, induction of killer cell activity [lymphokine-activated killer (LAK) and natural killer (NK) cells] and interferon gamma production, and proliferation of human interleukin-2-dependent cell lines.
Administration of aldesleukin in animals and humans produces multiple immunological effects in a dose-dependent manner. These effects include activation of cellular immunity and the production of cytokines including tumor necrosis factor, IL-1, and interferon gamma. In vivoexperiments in murine melanoma and sarcoma tumor models have shown inhibition of tumor growth.
12.2 Pharmacodynamics
Dose-dependent immunological effects including activation of cellular immunity with lymphocytosis, eosinophilia, and thrombocytopenia, and the production of cytokines including tumor necrosis factor, IL-1, and gamma interferon were observed following administration of aldesleukin in animals and humans.
Aldesleukin exposure-response relationships and the time course of pharmacodynamic response are unknown.
12.3 Pharmacokinetics
Aldesleukin serum concentrations change proportionally with the Proleukin dosage.
Distribution
Aldesleukin is rapidly distributed (distribution half-life of 13 minutes) into the extravascular space following a Proleukin intravenous infusion.
Elimination
The serum elimination half-life aldesleukin is 85 minutes in patients with cancer following a 5-minute intravenous infusion of Proleukin. The mean clearance rate of aldesleukin is 268 mL/min.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies.
In clinical studies, using an enzyme-linked immunosorbent assay (ELISA), low titers of anti-aldesleukin antibodies were observed in 74% (57 of 77) of patients with metastatic renal cell carcinoma treated with an every 8-hour Proleukin regimen and in 66% (33 of 50) of patients with metastatic melanoma treated with a variety of intravenous regimens. In a separate study in 13 patients, following the first cycle of therapy, the geometric mean aldesleukin exposure (AUC) on Day 15 compared to Day 1 increased by 68% in 11 patients who developed anti-aldesleukin antibodies while no change was observed in the 2 antibody-negative patients. Overall, neutralizing antibodies were detected in 1 patient. Based on these data, the clinical relevance of anti-aldesleukin antibodies could not be assessed.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Metastatic Renal Cell Cancer
The efficacy of Proleukin was evaluated in two hundred fifty-five patients with metastatic renal cell carcinoma (mRCC) in 7 clinical studies conducted at 21 institutions (mRCC studies). Eligible patients had an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0 or 1 and normal organ function as determined by cardiac stress test, pulmonary function tests, and creatinine ≤1.5 mg/dL. Studies excluded patients with brain metastases, active infections, organ allografts, and diseases requiring steroid treatment. Not all patients in these studies received the recommended Proleukin dosing regimen.
The major efficacy outcome measure was objective response rate (ORR) determined by investigator assessment per ECOG response criteria for solid tumors (1982).
Efficacy results are summarized in Table 5.
Table 5: Proleukin Efficacy Results in mRCC Studies Proleukin
(n=255)- * Denotes ongoing responses
Objective Response Rate ORR (95% CI), % 15% (11, 20) Complete Response (CR), % 7% Partial Response (PR), % 8% Duration of response (months) Number of Patients Who Responded n= 37 Median (months) 54 Range (months) 3, 131 * 14.2 Metastatic Melanoma
The efficacy of Proleukin was evaluated in two hundred seventy patients with metastatic melanoma in 8 clinical studies conducted at 22 institutions (metastatic melanoma studies). Eligible patients had an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0 or 1 and normal organ function as determined by cardiac stress test, pulmonary function tests, and creatinine ≤1.5 mg/dL. Studies excluded patients with brain metastases, active infections, organ allografts, and diseases requiring steroid treatment. Not all patients in these studies received the recommended Proleukin dosing regimen.
Patients with metastatic melanoma received a median of 18 of the 28 scheduled doses of Proleukin during the first course of therapy.
The major efficacy outcome measure was objective response rate (ORR) determined by investigator assessment per ECOG response criteria for solid tumors (1982).
Efficacy results are summarized in Table 6.
Table 6: Proleukin Efficacy Results in Metastatic Melanoma Studies Proleukin
(n=270)- * Denotes ongoing responses
Objective Response Rate ORR (95% CI) 16% (12, 21) Complete Response (CR), % 6% Partial Response (PR), % 10% Duration of response (months) Number of Patients Who Responded n= 43 Median (months) (95% CI) 9 Range (months) 1, 122 * -
16 HOW SUPPLIED/STORAGE AND HANDLING
Proleukin ®(aldesleukin) for injection is supplied in single-dose vials. Each vial contains 22 million International Units (1.3 mg) of Proleukin.
NDC: 73776-022-01 Individually boxed single-dose vial
-
17 PATIENT COUNSELING INFORMATION
Inform patients or caregivers of the following risks of Proleukin:
Capillary Leak Syndrome
Advise patients about the risk of capillary leak syndrome and to inform their healthcare provider immediately if they develop new or worsening symptoms of hypotension, dyspnea, or edema [see Warnings and Precautions (5.1)].
Neurotoxicity
Advise the patient to inform their healthcare provider immediately if they develop mental status changes, speech difficulties, blindness, ataxia, hallucinations, agitation, or experienced a seizure [see Warnings and Precautions (5.2)].
Serious Infections Including Sepsis
Advise patients to inform their healthcare provider immediately if they develop signs of infection or sepsis including fever, chills, weakness, dyspnea [see Warnings and Precautions (5.3)].
Immune-mediated Adverse Reactions
Advise patients that Proleukin can cause immune-mediated adverse reactions and can exacerbate pre-existing autoimmune disease. These immune-mediated adverse reactions can occur in any organ system or tissue. Proleukin may also increase the risk of allograft rejection in transplant patients. Advise patients to contact their healthcare provider for any new or worsening signs or symptoms [see Warnings and Precautions (5.5)] .
Hypothyroidism, sometimes preceded by hyperthyroidism, has been reported following Proleukin treatment. Evaluate thyroid function at baseline and periodically during treatment and initiate thyroid replacement therapy as clinically indicated.
Hyperglycemia and/or diabetes mellitus has been reported during Proleukin therapy. Monitor patients for hyperglycemia and initiate treatment with insulin as clinically indicated.
Serious Manifestations of Eosinophilia
Advise patients to inform their healthcare provider immediately if they develop serious symptoms of eosinophilia including new severe rash or dyspnea [see Warnings and Precautions (5.7)].
Delayed Adverse Reactions to Iodinated Contrast Media
Advise patients to inform their health care provider that they received Proleukin prior to undergoing imaging that requires iodinated contrast material [see Warnings and Precautions (5.8)].
Embryo-Fetal Toxicity
Advise females of reproductive potential to use effective contraception during treatment with Proleukin [see Warnings and Precautions (5.6), Use in Specific Populations (8.1, 8.3)] .
Proleukin may cause fetal harm. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.6), Use in Specific Populations (8.1, 8.3)] .
Lactation
Advise females not to breastfeed during treatment with Proleukin [see Use in Specific Populations (8.2)] .
Hypersensitivity
Advise patients to inform their healthcare provider if they develop signs and symptoms of hypersensitivity reactions [see Warnings and Precautions (5.9)].
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Iovance Biotherapeutics Manufacturing LLC
Philadelphia, PA 19112
U.S. License No. 2353At
Boehringer Ingelheim Pharma
Biberach/Riss, GermanyFor additional information, contact Iovance Biotherapeutics Manufacturing LLC. 1-844-845-IOVA (1-844-845-4682)
PROLEUKIN is a registered trademark.
© Iovance Biotherapeutics Manufacturing LLC.
-
PRINCIPAL DISPLAY PANEL - 1.3 mg Vial Carton
NDC: 73776-022-01
Proleukin ®
(aldesleukin)for injection
22 million
International Units/Vial
(1.3 mg/vial)Single-Dose Vial Discard Unused Portion
For Intravenous Infusion After Dilution
RefrigerateRx only
IOVANCE
BIOTHERAPEUTICS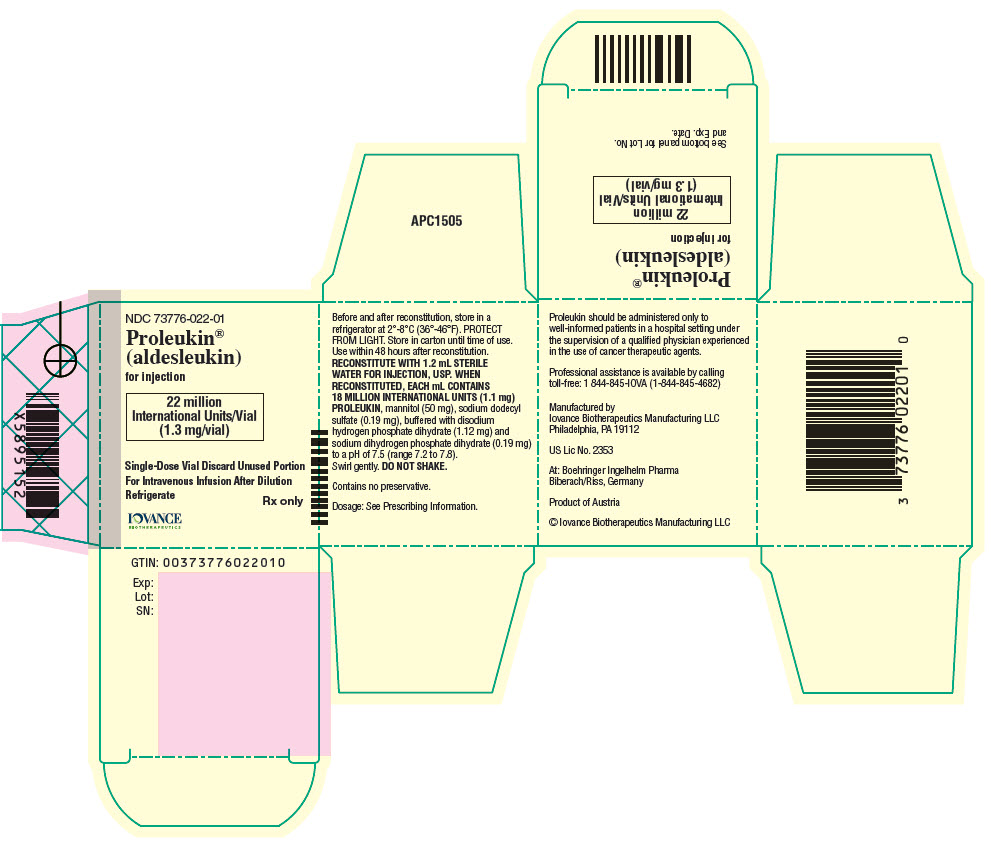
-
INGREDIENTS AND APPEARANCE
PROLEUKIN
aldesleukin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73776-022 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALDESLEUKIN (UNII: M89N0Q7EQR) (ALDESLEUKIN - UNII:M89N0Q7EQR) ALDESLEUKIN 1.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73776-022-01 1 in 1 CARTON 07/15/2024 1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103293 05/05/1992 Labeler - Iovance Biotherapeutics, Inc (962508433) Establishment Name Address ID/FEI Business Operations Boehringer Ingelheim Pharma GmbH and Co. KG 340700520 manufacture(73776-022) , analysis(73776-022)
Trademark Results [PROLEUKIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PROLEUKIN 86330987 4761828 Live/Registered |
CLINIGEN HOLDINGS LIMITED 2014-07-08 |
 PROLEUKIN 85199293 not registered Dead/Abandoned |
Novartis Vaccines & Diagnostics, Inc. 2010-12-16 |
 PROLEUKIN 79062942 3639936 Dead/Cancelled |
Novartis AG 2008-08-07 |
 PROLEUKIN 76532544 2857698 Dead/Cancelled |
Chiron Corporation 2003-07-25 |
 PROLEUKIN 73523710 1369770 Live/Registered |
CETUS CORPORATION 1985-02-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.