CUTAQUIG- immunoglobulin g solution
Cutaquig by
Drug Labeling and Warnings
Cutaquig by is a Other medication manufactured, distributed, or labeled by Octapharma USA Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CUTAQUIG safely and effectively. See full prescribing information for CUTAQUIG.
CUTAQUIG® (Immune Globulin Subcutaneous (Human) - hipp),
16.5% solution
Initial U.S. Approval: 2018WARNING: THROMBOSIS
See full prescribing information for complete boxed warning
Thrombosis may occur with immune globulin products, including CUTAQUIG. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors.
For patients at risk of thrombosis, administer CUTAQUIG at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
INDICATIONS AND USAGE
CUTAQUIG (Immune Globulin Subcutaneous (Human) - hipp) is a 16.5% immune globulin solution for subcutaneous infusion indicated for treatment of primary humoral immunodeficiency (PI) in adults. ( 1 )
DOSAGE AND ADMINISTRATION
For subcutaneous use only
Before switching to CUTAQUIG, obtain the patient’s serum IgG trough level to guide subsequent dose adjustments.
Dose ( 2.1 )
Switching from IGIV to CUTAQUIG: calculate the dosing by using a dose conversion factor (1.40); Switching from other IGSC: dosing should be the same as for previous IGSC.
Weekly : Start CUTAQUIG one week after last IGIV infusion
Initial weekly dose = Previous IGIV dose (in grams) x 1.40 No. of weeks between IGIV doses Adjust dosing according to patient’s pharmacokinetics and clinical response. ( see Dose Adjustment )
Administration ( 2.3 ):
Administer at regular weekly interval.
Infusion Parameters* Infusion Number 1st - 6th 7th and above Volume (mL/site) ≤ 25 gradually increase to max of 40 Rate (mL/hr/site) ≤ 20 ≤ 25 * As tolerated
Infusion sites: up to 6 infusions sites simultaneously, with at least 2 inches between sites. Rotate sites for each administration. ( 2.3 )
DOSAGE FORMS AND STRENGTHS
CUTAQUIG is a solution containing 16.5% IgG (165 mg/mL) for subcutaneous infusion. ( 3 )
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Hypersensitivity and anaphylactic reactions can occur with CUTAQUIG. IgA-deficient patients with anti-IgA antibodies are at greater risk of severe reactions. ( 5.1 )
Thrombosis may occur with CUTAQUIG. ( 5.2 )
Falsely elevated blood glucose readings may occur during and after the infusion of CUTAQUIG with some glucometer and test strip systems. ( 5.3 )
Aseptic meningitis syndrome (AMS) may occur within 2 days of treatment. ( 5.4 )
Monitor patients for signs and symptoms of renal dysfunction. Monitor blood urea nitrogen, serum creatinine, and urine output in patients at risk of acute renal failure. ( 5.5 )
Monitor for clinical signs and symptoms of hemolysis, especially in patients with pre-existing anemia and/or cardiovascular or pulmonary compromise. ( 5.6 )
Monitor for pulmonary adverse reactions (transfusion-related acute lung injury, TRALI). ( 5.7 )
CUTAQUIG is made from human plasma and may carry a risk of transmitting infectious agents, e.g. viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. ( 5.8 )
ADVERSE REACTIONS
The most common adverse reactions (≥ 5% of study subjects) were local infusion site reactions (such as redness, swelling, itching), headache, fever, diarrhea, dermatitis, asthma, and skin abrasion. ( 6 )
To report SUSPECTED ADVERSE REACTIONS, contact Octapharma USA Inc. at 1-866-766-4860 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
The passive transfer of antibodies may transiently interfere with the immune response to live virus vaccines, such as measles, mumps, varicella and rubella ( 7.2 ) and may lead to misinterpretation of the results of serological testing. ( 7.1 )
_________________________________________________________________________________________________________________________
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
BOXED WARNING
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Thrombosis
5.3 Falsely Elevated Glucose Readings
5.4 Aseptic Meningitis Syndrome (AMS)
5.5 Renal Dysfunction/Failure
5.6 Hemolysis
5.7 Transfusion-related Acute Lung Injury (TRALI)
5.8 Transmittable Infectious Agents
5.9 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Serological Testing
7.2 Live Virus Vaccines
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNING: THROMBOSIS
- Thrombosis may occur with immune globulin products, including CUTAQUIG. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. [see Warnings and Precautions ( 5.2 ), Patient Counseling Information ( 17 ) ]
- For patients at risk of thrombosis, administer CUTAQUIG at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity. [see Warnings and Precautions ( 5.2 )]
-
1 INDICATIONS AND USAGE
CUTAQUIG (Immune Globulin Subcutaneous (Human) - hipp) is a 16.5% immune globulin solution for subcutaneous infusion (IGSC), indicated as replacement therapy for primary humoral immunodeficiency (PI) in adults. This includes, but is not limited to, common variable immunodeficiency (CVID), X-linked agammaglobulinemia, congenital agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
-
2 DOSAGE AND ADMINISTRATION
For subcutaneous use only . Do not inject into a blood vessel.
- Before receiving treatment with CUTAQUIG: Obtain the patient's serum Immunoglobulin G (IgG) trough level to guide subsequent dose adjustments (see Dose Adjustment ).
2.1 Dose
- Individualize the dose based on the patient’s pharmacokinetic and clinical response. Monitor serum IgG trough levels regularly to guide subsequent dose adjustments as needed (see Dose Adjustment ).
- Start CUTAQUIG treatment one week after the last IGIV/IGSC infusion.
Dose for patients switching to CUTAQUIG from IGIV:
- Ensure that patients have received Immune Globulin Intravenous (Human) (IGIV) treatment at regular intervals for at least 3 months.
- Establish the initial weekly dose of CUTAQUIG by converting the monthly IGIV dose into an equivalent weekly dose and increasing it using a dose adjustment factor.
- To calculate the initial weekly dose of CUTAQUIG, divide the monthly IGIV dose in grams by the number of weeks between IGIV infusions and then multiply this value with a Dose Adjustment Factor of 1.40.
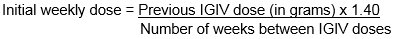
- To convert the dose (in grams) to milliliters (mL), multiply the calculated dose (in grams) by 6.
- Provided the total weekly dose is maintained, any dosing interval from daily up to weekly can be used and will result in systemic IgG exposure that is comparable to the previous IGIV treatment.
- On average, serum IgG trough levels were approximately 23% higher during CUTAQUIG administration compared to those obtained during prior IGIV therapy.
- To guide dose adjustments, see Table 1 under Dose Adjustment .
Dose for patients switching to CUTAQUIG from IGSC:
- Ensure that patients have received IGSC at regular intervals for at least 3 months before switching to CUTAQUIG.
- It is recommended to maintain the same weekly dosing (in grams) of CUTAQUIG that was used for the previous Immune Globulin Subcutaneous (Human) (IGSC) therapy (in grams).
- To convert the dose (in grams) to milliliters (mL), multiply the calculated dose (in grams) by 6.
- Obtain a trough IgG level before switching, monitor clinical response and check the trough IgG level 2 to 3 months after initiating CUTAQUIG.
- To guide dose adjustments, see Table 1 under Dose Adjustment , if the trough IgG level during CUTAQUIG administration differs from the trough IgG level obtained during treatment with the previously administered IGSC product or target trough level.
Over time the dose may need to be individualized for each patient, dependent on the pharmacokinetic and clinical response and on the desired IgG trough level. Measure the patient’s serum IgG trough level 2-3 months after switching to CUTAQUIG or after the last CUTAQUIG dose adjustment, in order to determine if a dose adjustment is necessary.
Calculate the difference between the patient’s target serum IgG trough level (in mg/dL) and the IgG trough level obtained during subcutaneous treatment with CUTAQUIG. Find this difference in column 1 of Table 1 and locate the corresponding adjustment amount of CUTAQUIG in mL/administration according to the body weight of the patient.
Use the patient’s clinical response as primary point to consider for any dose adjustment. Additional dose increments may be indicated based on the patient's clinical response (i.e., infection frequency and severity).
Table 1 Incremental adjustment (mL) of weekly CUTAQUIG dosing based on the calculated difference between actual IgG trough level and the target trough level for the patient*
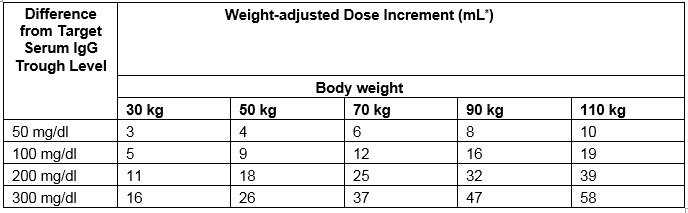
* Derived from a linear regression model of trough levels and weekly dose per kg body weight.
For example: a patient with body weight of 70 kg is treated weekly and has a trough level of 600 mg/dL, but the target trough level is 900 mg/dL The difference between the actual trough level (600) and the desired trough level (900) is plus 300 mg/dL. Therefore, the recommended increase in the weekly dose would be 37 mL.
Monitor the patient’s clinical response and periodically check trough IgG levels, and repeat dose adjustment as needed.
Measles Exposure
If a patient has been exposed to measles, it may be prudent to administer a dose of Immune Globulin Intravenous as soon as possible and within 6 days of exposure. A dose of 400 mg/kg should provide a serum level > 240 mIU/mL of measles antibodies for at least two weeks.
If a patient is at risk of future measles exposure and receives a dose of less than 245 mg/kg subcutaneously per week, the dose should be increased to 245 mg/kg.
2.2 Preparation
CUTAQUIG is a clear and colorless solution that may turn to slightly opalescent and pale yellow during storage. Do not use the solution if it appears cloudy or contains particulates.
- Use aseptic technique when preparing and administering CUTAQUIG.
- Prior to administration visually inspect each vial of CUTAQUIG for particulate matter, whenever the solution and container permit.
- Do not mix CUTAQUIG with other products.
- Do not dilute CUTAQUIG.
- Do not shake the solution.
- Check the labeling for the expiration date and do not use beyond this date.
- CUTAQUIG vials are for single use only. Discard any unused product after each infusion in accordance with local requirements.
- Do not freeze. Do not use frozen product.
- Record lot number in patient’s medical history.
2.3 Administration
CUTAQUIG is for subcutaneous use only. Do not inject into a blood vessel.
CUTAQUIG should be administered weekly by a healthcare provider, or by caregiver or self-administered by the patient after appropriate training.
CUTAQUIG may be infused in the following areas: abdomen, thigh, upper arm, and/or upper leg/hip area.
CUTAQUIG is intended for subcutaneous use using an infusion pump and compatible syringe(s).
CUTAQUIG may be infused simultaneously into up to 6 different sites. Infusion sites should be at least 2 inches (5 cm) apart. Rotate infusion sites between subsequent administrations.
Volume:
For patients not already on Immune Globulin Subcutaneous (Human) (IGSC) therapy, the maximum initial volume per injection site should not exceed 25 mL. From the 6 th infusion onwards the volume may be gradually increased to a maximum of 40 mL/site as tolerated.
Rate:
Maximum recommended flow rates per hour per infusion site are as follows:
First 6 infusions: 15-20 mL per hour per site.
Subsequent infusions: 25 mL per hour per site as tolerated.
Maximum recommended flow rates per hour for all sites combined :
First 6 infusions: 30 mL per hour for all sites combined.
From infusion number 7 onwards a gradual increase to 50 mL per hour for all sites combined, subsequently to 80 mL per hour for all sites combined, and if tolerated a further gradual increase to up to 100 mL per hour for all sites combined may be possible.
Table 2 Infusion Volumes and Infusion Rates
Infusion parameters* Infusion Number 1 st -6 th 7 th and above Volume (mL/site) ≤ 25 gradually increase to max of 40 Rate (mL/hr/site) ≤ 20 25 Rate (mL/hr/all sites combined) 30 Gradually increase to 50 mL, then to 80 mL; if well tolerated, use a max of 100 mL * As tolerated
Administration/Handling instructions
CUTAQUIG is for subcutaneous use only. Do not inject into a blood vessel.
Follow the administration guidance below and use aseptic technique when administrating CUTAQUIG.
Figure 1
1. Getting ready for infusion
- Choose and prepare a clean work area.
- Gather your infusion equipment:
Infusion pump and compatible syringe(s).
Needle or needleless transfer device (for drawing up product from the vial).
Infusion set (varies according to manufacturer’s instructions).
Infusion tubing and Y-connector (if required).
Ancillary supplies: disinfectant wipes, gauze or transparent dressing, tape and sharps container.
Patient’s treatment diary/log book.
- Wash your hands thoroughly and let them dry ( Figure 1 ).

Figure 2
2. Checking and opening the vials
- If taken out of the refrigerator, allow products to reach room temperature (≤ 25°C / 77°F).
- Inspect each vial carefully:
Check that labelled dose is correct and based on prescription.
Check that the expiration date has not been passed.
Check the appearance of the solution: do not use the solution if it is cloudy or contains particles.
Make sure the protective cap is not broken or missing.
- Remove the protective cap.
- Disinfect the rubber stopper by using a sterile wipe and allow it to dry ( Figure 2).
Figure 3
3. Preparing and filling the syringe
- Open sterile syringe and needle or needleless transfer device.
- If a needleless transfer device is used, follow the instructions of the device manufacturer.
- If the transfer is done using needle and syringe follow the instructions below:
Attach the needle to the syringe with a screw action.
Draw back on the plunger to fill the syringe with air which should be roughly equal to the amount of solution needed from the vial.
Insert the needle into the center of the vial stopper and turn the vial upside down. To avoid foaming, ensure that the tip of the needle is not in the solution, and then inject the air.
Next, move the needle so that the tip is in the solution, and then slowly draw up the desired volume of CUTAQUIG ( Figure 3 ).
Withdraw the needle from the vial.
This procedure might be repeated if you need to use more than one vial.
When finished, remove the needle and dispose of it into the sharps bin and proceed to the next step.
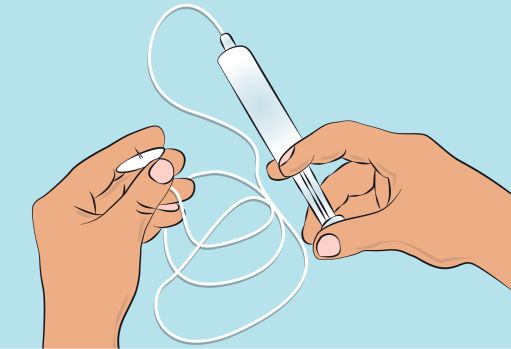
Figure 44. Preparing the infusion pump and tubing
- Follow the manufacturer’s instructions for preparing the infusion pump.
- Prime the administration tubing according to manufacturer’s instruction to eliminate all remaining air ( Figure 4 ).
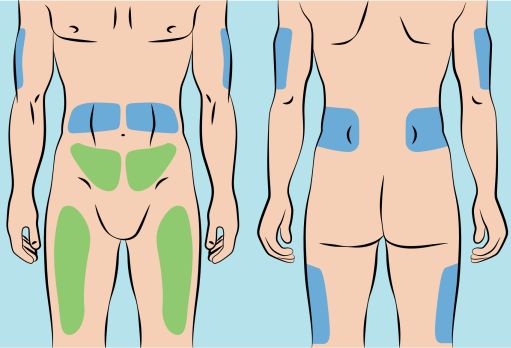
Figure 5
5. Preparing the infusion site(s) and inserting the infusion needle(s)
- CUTAQUIG can be infused in the following areas: abdomen, thigh, upper arm, and/or upper leg/hip area ( Figure 5 ).
- The number and location of injection sites depends on the volume of the total dose. The infusion sites should be at least 2 inches apart. A maximum of 6 infusion sites can be used simultaneously.
- Rotate sites between infusions.
- Avoid inserting the needle into scars, tattoos or injured/inflamed skin areas and inspect skin for signs of infection.
- Clean your skin at your selected infusion site(s) with an antiseptic skin wipe starting at the center and working outward in a circular motion, and allow each site to dry before proceeding.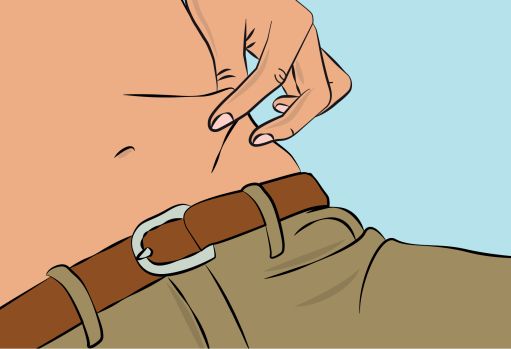
Figure 6- Pinch the skin between your thumb and forefinger around the injection site ( Figure 6 ) and insert the needle into the subcutaneous tissue ( Figure 7 ). The angle of the needle will depend on the type of infusion set being used.
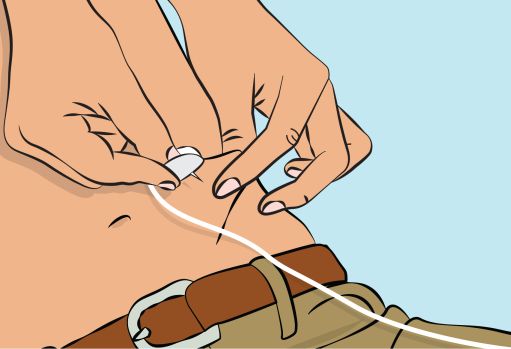
Figure 7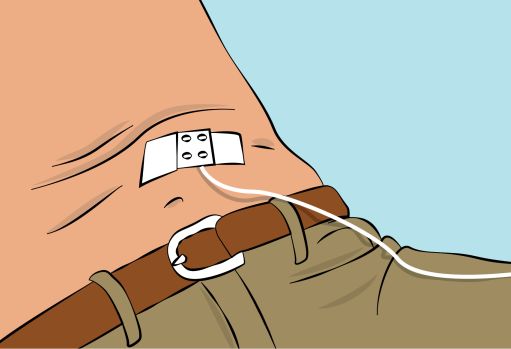
Figure 8- Secure the needle in place by applying sterile gauze and tape or transparent dressing ( Figure 8 ). 6. Checking the infusion
- Check needle placement by pulling back on the syringe plunger. There should not be any blood return in the tubing.
- If blood return is seen, remove needle and restart from step 5 with new tubing at a different location.
7. Starting the infusion
- Start the infusion. If an infusion pump is used for infusion, follow the manufacturer’s instructions.
8. Recording the infusion
- On each vial of CUTAQUIG, you will find a peel-off portion of the label with the batch number details. Stick this label in your patient’s treatment diary or infusion log book. Record details of the dose, date, time, infusion site location and any infections, side effects or other comments.
9. After infusion is complete
- Gently remove the needle(s) and immediately place into the sharps bin.
- If necessary press a small piece of gauze on the needle site and apply a dressing.
- Discard all used disposable supplies as well as any unused product and the empty vial(s) as recommended by your healthcare provider and according to local requirements.
- If using a pump, clean and store it according to the manufacturer’s instructions.
For self-administration, provide the patient and caregiver with instructions and appropriate training for infusion at home or other appropriate setting. Verify that the patient or caregiver is capable of self-administration using appropriate technique.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
CUTAQUIG is contraindicated:
- In patients who have had an anaphylactic or severe systemic reaction to the subcutaneous administration of human immune globulin or to any of the components of CUTAQUIG such as Polysorbate 80.
- In IgA-deficient patients with antibodies against IgA and a history of hypersensitivity to human globulin treatment.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur with CUTAQUIG, even in patients who tolerated previous treatment with human immune globulin. If a hypersensitivity reaction occurs, discontinue the CUTAQUIG infusion immediately and initiate appropriate treatment. Have epinephrine immediately available to treat any severe acute hypersensitivity reaction.
IgA-deficient patients with known anti-IgA antibodies have a higher risk of developing potentially severe hypersensitivity and/or anaphylactic reactions (including anaphylaxis and shock) with administration of CUTAQUIG. CUTAQUIG contains ≤ 0.6 mg of IgA /mL.
5.2 Thrombosis
Thrombosis may occur following treatment with immune globulin products, including CUTAQUIG. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, such as those with cryoglobulins, fasting chylomicronemia/markedly high triglycerides, or monoclonal gammopathies. For patients at risk of thrombosis, administer CUTAQUIG at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. [see Boxed Warning , Patient Counseling Information ( 17 )]
5.3 Falsely Elevated Glucose Readings
Blood Glucose Testing: Some types of blood glucose testing systems (for example, those based on the glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase methods) falsely interpret the maltose contained in CUTAQUIG as glucose. This may potentially result in falsely elevated glucose readings and, consequently, in the inappropriate administration of insulin, resulting in life-threatening hypoglycemia. Also, cases of true hypoglycemia may go untreated if the hypoglycemic state is masked by falsely elevated glucose readings. Accordingly, when administering CUTAQUIG, measure blood glucose with a glucose-specific method. The product information of the blood glucose testing system, including that of the test strips, should be carefully reviewed to determine if the system is appropriate for use with maltose-containing parenteral products. If any uncertainty exists, contact the manufacturer of the testing system to determine if the system is appropriate for use with maltose-containing parenteral products.
5.4 Aseptic Meningitis Syndrome (AMS)
Aseptic Meningitis Syndrome can occur with CUTAQUIG. AMS has been reported after the use of human immune globulin administered intravenously and subcutaneously. It usually begins a few hours to 2 days following treatment and occurs more frequently in females than in males.
AMS is characterized by the following signs and symptoms: severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea and vomiting. Cerebrospinal fluid (CSF) studies frequently show pleocytosis up to several thousand cells per mm 3 , predominantly from the granulocytic series, and elevated protein levels up to several hundred milligram/dL, but negative culture results. To rule out other causes of meningitis, conduct a thorough neurological examination on patients showing such symptoms and signs, including CSF studies. Discontinuation of immunoglobulin treatment has resulted in remission of AMS within several days without sequelae.
5.5 Renal Dysfunction/Failure
Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis and death may occur with use of human immune globulin, especially those containing sucrose. CUTAQUIG does not contain sucrose. Ensure that patients are not volume-depleted before administration of CUTAQUIG.
In patients at risk of developing renal dysfunction because of any degree of preexisting renal insufficiency or predisposition to acute renal failure (such as diabetes mellitus, age greater than 65 years, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs) monitor renal function and consider lower, more frequent dosing [see Dosage and Administration ( 2 ), Patient Counseling Information ( 17 )].
Periodic monitoring of renal function and urine output is particularly important in patients judged to have a potential increased risk of developing acute renal failure. Assess renal function, including measurements of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of CUTAQUIG and again at appropriate intervals thereafter. If renal function deteriorates, consider discontinuing CUTAQUIG.
5.6 Hemolysis
IgG products, including CUTAQUIG, can contain blood group antibodies that may act as hemolysins and induce in vivo coating of red blood cells (RBCs) with immunoglobulin, causing a positive direct antiglobulin (Coombs’) test result. Delayed hemolytic anemia can develop subsequent to immune globulin therapy due to enhanced RBC sequestration, and acute hemolysis, consistent with intravascular hemolysis, has been reported.
Monitor CUTAQUIG recipients for clinical signs and symptoms of hemolysis, particularly patients with risk factors (such as non-O blood group, administration of high IgG doses (≥ 2g/kg BW)). Underlying inflammatory state in a patient may increase the risk of hemolysis but its role is uncertain. Consider appropriate confirmatory laboratory testing if signs and symptoms of hemolysis are present after CUTAQUIG infusion.
5.7 Transfusion-related Acute Lung Injury (TRALI)
Non-cardiogenic pulmonary edema may occur in patients administered human immune globulin products. TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Typically, it occurs within 1 to 6 hours following transfusion.
Monitor patients for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies in both the product and patient’s serum. Patients with TRALI may be managed using oxygen therapy with adequate ventilatory support.
5.8 Transmittable Infectious Agents
Because CUTAQUIG is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens. No cases of transmission of viral diseases or CJD have been associated with the use of CUTAQUIG. All infections suspected by a physician to have possibly been transmitted by CUTAQUIG should be reported to Octapharma USA Inc. at 1-866-766-4860.
5.9 Interference with Laboratory Tests
After infusion of CUTAQUIG, the transitory rise of the various passively transferred antibodies in the patient’s blood may yield false positive serological test results, with the potential for misleading interpretation. Passive transmission of antibodies to erythrocyte antigens (e.g.. A, B, and D) may cause a positive direct or indirect antiglobulin (Coombs’) test.
-
6 ADVERSE REACTIONS
The most common adverse reactions (AR) observed in ≥ 5% of study subjects were local infusion site adverse reactions (such as redness, swelling, and itching), and, as systemic adverse reactions, headache, fever, diarrhea, dermatitis, asthma, and skin abrasion.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical safety data are based on an open-label, single arm, prospective, multicenter major effectiveness study of CUTAQUIG in subjects with primary humoral immunodeficiency (PI), previously treated with Immune Globulin Intravenous (Human) (IGIV) for at least 6 months. This study was conducted in Europe and North America.
Overall, the 61 subjects in the Safety Analysis Set received 3497 infusions in the study, with a mean of 57 infusions administered per subject. The average dose of CUTAQUIG used per subject was 0.185 g/kg in adult subjects, 0.135 g/kg in young children, 0.160 g/kg in older children and 0.172 g/kg in adolescents.
Excluding infections, a total of 50 subjects (82%) experienced 950 adverse reactions (ARs, defined as adverse events occurring during or within 72 hours of infusion or any adverse events otherwise causally related). Of the 950 ARs 814 were infusion site reactions. Forty-three subjects (70.5%) had at least one systemic AR. The proportion of infusions with adverse reactions was 0.27.
Local reactions were the most common ARs and were experienced by 46 subjects (75.4%). The rate of infusion site reaction per infusion was 0.23. Most local ARs were either mild (transient AR causing discomfort but not interfering with routine activities [89.4%]) or moderate (AR sufficiently discomforting to interfere with routine activities [10.3%]) in intensity.
Table 3 summarizes adverse reactions occurring in ≥ 5% of subjects (defined as adverse events occurring during or within 72 hours of infusion or any adverse events otherwise considered causally related event occurring within the study period).
Table 3 Adverse reactions* in ≥ 5% of subjects and rate per infusion
ARs Number (%) of subjects (N=61) Number (rate**) of ARs (N=3497) Local reaction 46 (75.4) 814 (0.23) Systemic ARs Headache 7 (11.5%) 8 (0.0023) Fever 5 (8.2%) 6 (0.0017) Diarrhea 5 (8.2%) 8 (0.0023) Dermatitis 5 (8.2%) 6 (0.0017) Asthma 4 (6.6%) 5 (0.0014) Skin abrasion 4 (6.6%) 4 (0.0011) * Excluding infections.
** Rate = total number of adverse reactions divided by total number of infusions.
In the study, the most frequent infusion site reactions were redness, swelling, and itching. During the study period the incidence of local reactions decreased over time from 38% of infusions triggering an infusion site reaction during the first 4 infusions to around 15% of infusions with an infusion site reaction during the last 4 infusions.
No adverse events (AEs) led to withdrawal of subject from the study and none led to discontinuation of the study medication.
6.2 Postmarketing Experience
There is no post-marketing experience with CUTAQUIG. The following adverse reactions have been identified during post-approval use of immune globulins. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Table 4 summarizes adverse reactions that have been reported during post-marketing use of immune globulin products and may also occur after CUTAQUIG administration:
Table 4 Adverse reactions reported during post-marketing use of immune globulin products
MeDRA System Organ Class (SOC) Adverse reaction Blood and lymphatic system disorders Pancytopenia, leukopenia, hemolytic anemia Immune system disorders Anaphylactic reaction, hypersensitivity reaction, allergic reaction, angioneurotic edema, face edema Psychiatric disorders Agitation Nervous system disorders Loss of consciousness, cerebrovascular accident, aseptic meningitis, seizures, migraine, tremor, paresthesia, dizziness Cardiac disorders Cardiac arrest, tachycardia, palpitations Vascular disorders Thromboembolism, thrombosis, circulatory collapse, hypertension, hematoma Respiratory, thoracic and mediastinal disorders Transfusion-related acute lung injury, acute respiratory distress syndrome, respiratory failure, pulmonary embolism, apnea, cyanosis, hypoxia, pulmonary edema, bronchospasm, dyspnea, cough, wheezing Gastrointestinal disorders Hepatic function abnormal, nausea, vomiting, abdominal distension, abdominal pain upper Skin and subcutaneous tissue disorders Stevens-Johnson syndrome, epidermolysis, erythema multiforme, eczema, urticaria, rash (erythematous), alopecia, skin discoloration, skin mass, skin reaction, skin/infusion site ulceration, skin/infusion site necrosis Musculoskeletal and connective tissue disorders Back pain, arthralgia, pain in extremity, myalgia Renal and urinary disorders (Acute) renal failure General disorders and administration site condition Chills, chest pain, chest discomfort, hot flush, flushing, hyperhidrosis, fatigue, influenza-like illness, malaise Investigations Hepatic enzyme increased, coombs test positive, free hemoglobin present, hemoglobin increased, haptoglobin decreased To report SUSPECTED ADVERSE REACTIONS, contact Octapharma USA Inc. at 1-866-766-4860 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
7 DRUG INTERACTIONS
7.1 Serological Testing
Various passively transferred antibodies in immunoglobulin preparations may lead to misinterpretation of results of serological testing.
7.2 Live Virus Vaccines
The passive transfer of antibodies with immunoglobulin administration may interfere with the response to live virus vaccines such as measles, mumps, rubella, and varicella. Inform the immunizing physician of recent therapy with CUTAQUIG so that appropriate precautions can be taken. [See Patient Counselling Information ( 17 )]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
No human data are available to indicate the presence or absence of drug-associated risk. Animal reproduction studies have not been conducted with CUTAQUIG. It is not known whether CUTAQUIG can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Immune globulins cross the placenta from maternal circulation increasingly after 30 weeks of gestation. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk summary
No human data are available to indicate the presence or absence of drug associated risk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CUTAQUIG and any potential adverse effects on the breastfed infant from CUTAQUIG or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of CUTAQUIG have not been established in patients under 17 years of age. There are only limited data available on the safety and efficacy of CUTAQUIG administration in pediatric patients. CUTAQUIG was evaluated in 23 pediatric subjects (15 children, 8 adolescents) with primary humoral immunodeficiency (PI).
8.5 Geriatric Use
Clinical studies of CUTAQUIG did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Three study subjects enrolled in the clinical trial were 65 years and over. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
CUTAQUIG (Immune Globulin Subcutaneous (Human) - hipp), is a solvent/detergent (S/D)-treated, sterile preparation of highly purified immunoglobulin G (IgG) derived from large pools of human plasma. CUTAQUIG is a solution for injection to be administered subcutaneously.
This preparation contains approximately 165 mg of protein per mL (16.5%), of which not less than 96% is normal human immunoglobulin G. CUTAQUIG contains not more than 3% aggregates, not less than 94% monomers and dimers, and not more than 3% fragments. On average, the product contains ≤ 0.6 mg of IgA /mL.
The sodium content of the final solution is not more than 30 mmol/L and the pH is between 5.0 and 5.5. The osmolality is 310 - 380 mOsmol/kg.
The manufacturing process for CUTAQUIG isolates IgG without additional chemical or enzymatic modification, and the Fc portion is maintained intact. CUTAQUIG contains the IgG antibody activities present in the donor population. IgG subclasses are fully represented with the following approximate percents of total IgG: IgG1 is 70%, IgG2 is 25%, IgG3 is 3% and IgG4 is 2%.
CUTAQUIG contains a broad spectrum of IgG antibodies against bacterial and viral agents that are capable of opsonization and neutralization of microbes and toxins. It contains maltose (79 mg/mL), but no preservatives or sucrose.
All units of human plasma used in the manufacture of CUTAQUIG are provided by FDA-approved blood and plasma establishments, and are tested by FDA-licensed serological tests for HBsAg, antibodies to HCV and HIV and Nucleic Acid Test (NAT) for HCV and HIV-1 and found to be non-reactive (negative).
The product is manufactured by the cold ethanol fractionation process followed by ultrafiltration and chromatography. The manufacturing process includes treatment with an organic S/D mixture composed of tri-n-butyl phosphate (TNBP) and Octoxynol. The CUTAQUIG manufacturing process shows significant viral reduction in in vitro studies ( Table 5 ). These reductions are achieved through a combination of process steps including cold ethanol fractionation, S/D treatment and pH 4 treatment.
Table 5 Pathogen Reduction During CUTAQUIG Manufacturing
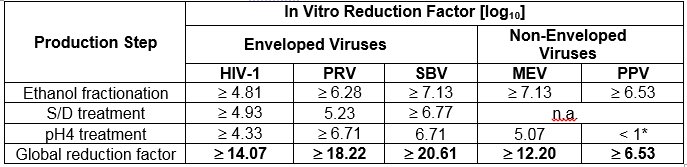
* Not calculated for global LRF
HIV-1: Human Immunodeficiency Virus - 1
PRV: Pseudorabies Virus, model virus for e.g. Hepatitis B Virus (HBV)
SBV: Sindbis Virus model virus for Hepatitis C Virus (HCV)
MEV: Mouse Encephalomyelitis Virus, model virus for Human Parvovirus B19
PPV: Porcine Parvovirus, model virus for Hepatitis A Virus (HAV)
n.a.: not applicable
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CUTAQUIG supplies a broad spectrum of opsonizing and neutralizing Immunoglobulin G (IgG) antibodies against a wide variety of bacterial and viral agents. It has a distribution of immune globulin subclasses closely proportional to that in native human plasma. The mechanism of action in primary humoral immunodeficiency (PI) has not been fully elucidated; however adequate doses may restore abnormally low immune globulin G levels to the normal range and thus help in preventing infections.
12.2 Pharmacodynamics
CUTAQUIG contains mainly IgG with a broad spectrum of antibodies against various infectious agents reflecting the IgG activity found in the donor population. CUTAQUIG which is prepared from pooled material from not less than 1000 donors, has an IgG subclass distribution similar to that of native human plasma. Adequate doses of Immune Globulin Subcutaneous (Human) (IGSC) can restore an abnormally low IgG level to the normal range. Standard pharmacodynamic studies were not performed.
12.3 Pharmacokinetics
A pharmacokinetic (PK) sub-study was conducted in 18 adult subjects who were enrolled in the 61-subject safety and efficacy study. Blood samples for PK study were collected prior to switching to CUTAQUIG (IGIV profile: PK IV ), after the 11 th infusion of CUTAQUIG (first SC profile: PK SC1 ) and after the 28th infusion of CUTAQUIG (second SC profile: PK SC2 ). The objective of the PK sub-study was to compare the AUCs following the IV and SC administration. At steady state, the geometric mean of the ratio (SC2:IV) was 1.02, (90% CI: 0.96, 1.08), indicating comparable exposure between IGSC and IGIV treatment (standardized to a 7-day period). Dose conversion factors from IGIV to IGSC of individual subjects ranged from 1.24 to 1.89 (mean: 1.40).
Serum IgG and IgG subclass trough levels were nearly constant during the IGSC phase of the study, with higher mean levels after SC treatment compared with those following IGIV. At the end of the IGIV period, trough levels ranged from 5.8 g/L to 13.9 g/L. Over the entire IGSC treatment period individual trough levels of total IgG ranged between 6.1 g/L to 24.0 g/L.
Table 6 summarizes the Key PK parameters for CUTAQUIG.
Table 6 Key Pharmacokinetic Parameters for CUTAQUIG and IGIV in Adults
Parameter
[Arithmetic Mean (SD)]IGIV
(n=18)CUTAQUIG
(n=18)C max [g/L] 19.7 (5.6) 14.0 (4.4) C min [g/L] 10.5 (2.6) 12.0 (3.5) T max [h] # 2.9 (2.1 - 69.5) 49.3 (1.8 - 98.3) AUC tau [g*hr/L] 2182 (692)* 2408 (673) AUC tau [mg*day/dL] 9091 (2881)* 10031 (2804) CL + (mL/day/kg) 1.5 (0.4) 1.9 (0.5) Actual IgG Dose per kg Body Weight and Week (g/kg/week) 0.135 (0.059) 0.188 (0.083) # T max is presented as Median (range)
* standardized to a 7-day period
+ apparent clearance (CL/F) for CUTAQUIG (F = bioavailability)
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies were conducted with CUTAQUIG to assess carcinogenesis, mutagenesis or impairment of fertility.
13.2 Animal Toxicology and/or Pharmacology
Safety of CUTAQUIG has been demonstrated in several standard nonclinical toxicology studies (local tolerance in rabbits, cardiovascular and respiratory effects in dogs, thrombogenic potential in rabbits).
The product given subcutaneously to CD1 mice inoculated with Streptococcus pneumoniae showed a dose related improvement in survival.
TNBP and Octoxynol may be found in CUTAQUIG in trace amounts. In animals and in vitro studies, these compounds were not genotoxic and did not have mutagenic properties. They did not show teratogenic effects when administered to pregnant rabbits and rats during organogenesis.
-
14 CLINICAL STUDIES
The study was a prospective, open-label, non-controlled, single-arm, multicenter study to evaluate the pharmacokinetics (PK), efficacy, tolerability and safety of subcutaneous human immunoglobulin (CUTAQUIG) in subjects with primary humoral immunodeficiency (PI).
The study was conducted in 61 subjects (38 adult and 23 pediatric subjects < 16 years of age) who received weekly SC infusions with CUTAQUIG during a 12-week wash-in/wash-out period followed by a 12-month efficacy period during which efficacy, pharmacokinetics, safety, tolerability, and quality of life (QoL) parameters of CUTAQUIG were evaluated. At the time of data analysis, 47 subjects (35 adults; 12 pediatric subjects) had completed the study, 8 pediatric subjects were continuing in the study, and 6 subjects (3 adults; 3 pediatric subjects) had discontinued the study prematurely at the time of data analysis.
During the efficacy period the mean weekly dose was 175 mg/kg BW, with individual doses ranging from 60 to 390 mg/kg BW. The median duration of infusion per week was 1.5 hours.
All enrolled subjects (n=61) were included in the Safety Analysis Set and the Full Analysis Set (FAS). Four subjects were excluded from the Per-Protocol (PP) Set because they terminated early before the start of the primary treatment period.
Overall, 33 female subjects and 28 male subjects participated in this study. The youngest subject enrolled in the study was 2 years old and the oldest was 73 years old. The mean age in the adult group (16–75 yrs) was 46.6 years. Reported race was white for all but one subject, and all subjects were of not Hispanic/Latino ethnicity.
The main objective of the study was to assess the efficacy of CUTAQUIG in preventing serious bacterial infections (SBI defined as bacteraemia/sepsis, bacterial meningitis, osteomyelitis/septic arthritis, bacterial pneumonia and visceral abscess). This endpoint was considered successful if the upper bound of the 99% confidence interval for the rate of SBIs was < 1.0 per subject-year of follow up. This criterion was met, as no SBIs were reported at any time during the study.
Other endpoints of the study included, but were not limited to: the number of episodes of any other infections, along with type and severity of infection and time to resolution; number of days of use and annual rate of antibiotics; absence and number of days of absence from work/school/ kindergarten/day care; and hospitalisations due to infections and number of days and annual rate of hospitalisation.
Efficacy results are summarized in Table 7 .
Table 7 Summary of efficacy results (FAS set).
Number of subjects (efficacy period) 61 Total number of subject years
Infections
Annual rate of SBIs*
Annual rate of other infections per subject-year54.8
0 SBI per subject-year #
3.4 (Upper one-sided 95% confidence limit: 4.6)Systemic antibiotic use
Number of subjects (%)
Annual rate (treatment days per subject-year)
40 (65.6%)
39.6 (Upper one-sided 95% confidence limit: 62.7)Days out of work/school/kindergarten/day care due to infections
Number of days
Annual rate (days per subject-year)
134
2.6 (Upper one-sided 95% confidence limit: 4.7)Hospitalization due to infections
Number of days
Annual rate (days per subject-year)1
2
0.04 (Upper one-sided 95% confidence limit: 0.19)* Defined as bacterial pneumonia, bacteremia/septicemia, osteomyelitis/septic arthritis, bacterial meningitis, and visceral abscess.
# Upper one-sided 99% confidence limit: 0.08.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CUTAQUIG is supplied in 1 g, 1.65 g, 2 g, 3.3 g, 4 g or 8 g single-use vials.
Carton NDC Number Container NDC Number Size Grams Protein 68982-810-01
68982-810-02
68982-810-03
68982-810-04
68982-810-05
68982-810-0668982-810-81
68982-810-82
68982-810-83
68982-810-84
68982-810-85
68982-810-866 mL
10 mL
12 mL
20 mL
24 mL
48 mL1
1.65
2
3.3
4
8- Components used in the packaging of CUTAQUIG are not made with natural rubber latex.
- Store at +2°C to +8°C (36°F to 46°F) for up to 24 months from the date of manufacture. Within its shelf-life, the product may be stored at room temperature up to +25°C (77°F) for up to 6 months without being refrigerated again during this period, and must be discarded if not used after this.
- Keep the vial in the outer carton to protect it from light.
- Check the product expiration date on the vial label. Do not use beyond the expiration date.
- Do not freeze. Do not use frozen product.
- Do not mix with other products.
- Do not shake the vial.
- Use aseptic technique when preparing and administering CUTAQUIG. Prior to administration, visually inspect each vial for particulate matter, whenever the solution and container permit. Do not use if the solution is cloudy or contains particulates.
- The vial is for single-use only. Discard any unused product after each infusion, in accordance with local requirements.
-
17 PATIENT COUNSELING INFORMATION
Advise the patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Inform patients to immediately report the following signs and symptoms to their healthcare provider:
- Hypersensitivity reactions to CUTAQUIG (including hives, generalized hives, tightness of the chest, wheezing, low blood pressure, and anaphylaxis): Advise patients to have epinephrine immediately available to treat any acute severe hypersensitivity reaction and ensure patients and their caregivers are properly trained in its use. [see Warnings and Precautions ( 5.1 )].
- Pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, or numbness or weakness on one side of the body [see Warnings and Precautions ( 5.2 )].
- Severe headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea, and vomiting [see Warnings and Precautions ( 5.4 )].
- Decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath [see Warnings and Precautions ( 5.5 )].
- Fatigue, increased heart rate, yellowing of the skin or eyes, and dark-colored urine [see Warnings and Precautions ( 5.6 )].
- Severe breathing problems, lightheadedness, drops in blood pressure, and fever [see Warnings and Precautions ( 5.7 )].
Inform patients to be tested periodically to make sure they have the appropriate levels of IgG in their blood. These tests may result in adjustments to the CUTAQUIG dose.
Inform patients/caregivers that because CUTAQUIG is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent [see Warnings and Precautions ( 5.8 ), Description ( 11 )].
Inform patients that CUTAQUIG may interfere with the response to live virus vaccines (e.g., measles, mumps, rubella, and varicella) and to notify their immunizing physician of recent therapy with CUTAQUIG [see Drug Interactions ( 7.2 )].
Home treatment/Self administration
- If self-administration is deemed appropriate by the physician, it is highly important that the patient/caregiver receives clear instructions and thorough training on subcutaneous administration of CUTAQUIG. The patient/caregiver needs to demonstrate the ability to independently administer subcutaneous infusions and this should be documented.
- Ensure the patient/caregiver understands the importance of regular subcutaneous infusions to maintain appropriate steady state IgG levels.
- Instruct the patient/caregiver to keep a treatment diary/log where all information about each infusion should be clearly documented.
- Inform patients to interrupt or terminate the CUTAQUIG infusion if a hypersensitivity reaction occurs.
- Inform patients that they should be tested regularly to make sure they have the correct levels of CUTAQUIG (IgG) in their blood. These tests may result in adjustment to the CUTAQUIG dose.
- Instruct the patient/caregiver that local injection site reactions (such as swelling, redness, itching) are a common side effect of subcutaneous treatment, but also tell them to contact their healthcare provider if local reactions increase in severity or persist for more than a few days.
- Inform the patient that injection sites should be rotated between infusions and that CUTAQUIG is for subcutaneous infusion only.
Manufactured by:
Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Strasse 235
1100 Vienna, Austria
U.S. License No. 1646
Distributed by:
Octapharma USA Inc.
121 River Street, Suite 1201
Hoboken, NJ 07030
-
PATIENT PACKAGE INSERT
Patient Information
CUTAQUIG
(kew' ta kwig)
Immune Globulin Subcutaneous (Human) - hipp, 16.5% solution
Information for Patients
The following summarizes important information about CUTAQUIG. Please read it carefully before using CUTAQUIG and each time you get a refill, as there may be new information. This Patient Information does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions after reading this, ask your healthcare provider.
What is CUTAQUIG?
CUTAQUIG is a ready-to-use liquid solution of Immunoglobulin G (IgG), also called antibodies, which protect the body against infection. CUTAQUIG is used to treat patients with primary humoral immunodeficiency (PI).
There are many forms of PI. The most common types of PI result in an inability to make a very important type of protein called antibodies, which help the body fight off infections from bacteria or viruses. Regular administration of CUTAQUIG will help your body to fight bacteria and viruses that cause infections. CUTAQUIG is made from human plasma that is donated by healthy people. CUTAQUIG contains antibodies collected from these healthy people; these antibodies replace the missing antibodies in PI patients.
Who should NOT use CUTAQUIG?
Do not use CUTAQUIG if you have ever had a severe allergic reaction to immune globulin or other blood products.
Tell your healthcare provider if you:
- ever had any severe reaction to other immune globulin medicines.
- were told that you have a condition called IgA deficiency.
- have a history of heart or blood vessel disease.
- have had blood clots or “thick blood”.
- have been immobile for some time.
What should I tell my healthcare provider before using CUTAQUIG?
Talk to your healthcare provider about any medical conditions that you have or have had.
Tell your healthcare provider that you are taking CUTAQUIG before you get vaccination as vaccines may not work while you are taking CUTAQUIG.
Tell your healthcare provider about all of the prescription and non-prescription medicines you take, including over-the-counter medicines, dietary supplements, or herbal medicines.
Tell your healthcare provider if you are pregnant or plan to get pregnant, or if you are nursing because CUTAQUIG might not be right for you.
Tell your healthcare provider if you have diabetes. If you need to do glucose testing, your healthcare provider may tell you to use a different way to monitor your blood sugar levels on the day that you receive a CUTAQUIG infusion. Some types of blood glucose testing systems (so called glucometers) falsely interpret the maltose contained in CUTAQUIG as glucose. If you are uncertain ask your healthcare provider which glucose testing system you can use while using CUTAQUIG.
How should I use CUTAQUIG?
CUTAQUIG is given under the skin (subcutaneously). This type of infusion can be given at home by yourself or by your caretaker after appropriate training. Administration of CUTAQUIG should be done at regular weekly intervals. A detailed description for giving CUTAQUIG under the skin is given in the Instructions for Use at the end of this package insert. Only use CUTAQUIG by yourself after you have been instructed by your healthcare provider.
What are the possible or reasonably likely side effects of CUTAQUIG?
The most common side effects of CUTAQUIG are:
- Infusion site reactions (including but not limited to redness, swelling, itching, fluid in tissue, pain, mass, bruising)
- Headache
- Elevated body temperature
Call your healthcare provider or emergency department immediately if you have any of the following symptoms: difficulty breathing, chest tightness, itching, swelling of the face, rash, hives or dizziness. These could be signs of a serious allergic reaction.
Tell your healthcare provider immediately if you have any of the following symptoms. They could be signs of a serious drug reaction:
- Decreased urination, sudden weight gain, or swelling in the legs. These could be signs of a kidney problem.
- Bad headache with nausea, vomiting, stiff neck, fever, and sensitivity to light. These could be signs of meningitis, which is irritation of the lining around your brain.
- Pain, swelling, warmth, discoloration of an arm or leg, unexplained shortness of breath, chest pain, discomfort that worsens on deep breathing, unexplained rapid pulse, a lump in your legs or arms, or numbness or weakness on one side of the body. These could be signs of a blood clot.
- Chest pain or trouble breathing, or blue lips or extremities. These could be signs of a serious heart or blood problem.
- Brown or red urine, fast heart rate, yellow skin or eyes. These could be signs of a liver or heart problem.
- Fever over 100°F (38°C). This could be sign of an infection.
These are not all of the possible side effects from CUTAQUIG. Ask your healthcare provider for more information. You are encouraged to report side effects to Octapharma USA Inc. at 1-866-766-4860 or FDA at 1-800-FDA-1088.
Talk to your healthcare provider about any side effect that bothers you or that does not go away. You can ask your healthcare provider for more information on possible side effects.
Whenever giving yourself treatments at home, you should have another responsible person present to help treat side effects or get help if you have a serious adverse reaction occur. Ask your healthcare provider whether you should have rescue medications, such as antihistamines or epinephrine. If prescribed epinephrine for severe allergic reactions, make sure you receive adequate training from a healthcare provider for its proper use.
How should I store CUTAQUIG?
Keep CUTAQUIG in the outer carton to protect it from exposure to light.
Do not freeze CUTAQUIG.
You can store CUTAQUIG at 36°F- 46°F (+2°C to +8°C) for up to 24 months from the date of manufacture. Within its shelf-life, the product may be stored at room temperature up to 77°F (up to +25°C) for up to 6 months without being refrigerated again during this period, and must be discarded if not used after this.
Do not use CUTAQUIG after the expiration date printed on the vial.
Dispose all materials, including any unused CUTAQUIG, in an appropriate container.
What else should I know about CUTAQUIG?
Do not use CUTAQUIG for a medical condition for which it was not prescribed. Do not share CUTAQUIG with other people, even if they have the same diagnosis and symptoms that you have.
Resources at Octapharma available to patients
For more product information on CUTAQUIG, please visit www.cutaquig.com.
For more information on patient assistance programs that are available to you, please contact the Octapharma Patient Support Center at 1-800-554-4440.
Manufactured by
Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Strasse 235
1100 Vienna, Austria
U.S. License No. 1646
Distributed by
Octapharma USA, Inc.
121 River Street, Suite 1201
Hoboken, NJ 07030
cutaquig ® is a registered trademark of Octapharma.
Instructions for Use
CUTAQUIG
Immune Globulin Subcutaneous (Human) - hipp, 16.5% solution
Detailed patient handling instructions for administration of CUTAQUIG
CUTAQUIG is for subcutaneous use only. Do not inject into a blood vessel.
Use CUTAQUIG only after you have been properly instructed and trained by your healthcare provider.
Follow the administration guidance below step by step and use aseptic/sterile technique when administrating CUTAQUIG. Use gloves if you have been told to do so when preparing the infusion.
1. Prepare the necessary number of CUTAQUIG vials
- If stored in the refrigerator, put the vials at room temperature at least 90 minutes prior to infusion.
- Do not heat the vials or put them into the microwave.
- Do not shake the vials to avoid foaming.
Figure 1
2. Getting ready for infusion
- Choose and prepare a clean work area using antiseptic wipes or disinfecting solution.
- Gather your infusion equipment:
- Infusion pump and compatible syringe(s)
Needle or needleless transfer device (for drawing up product from the vial)
Infusion set (varies according to manufacturer’s instructions)
Infusion tubing and Y-connector (if required)
Ancillary supplies: disinfectant wipes, gauze or transparent dressing, tape and sharps container
Treatment diary and pen
- Wash and clean your hands thoroughly and let them dry as has been shown to you during the training ( Figure 1 ). You can wear gloves during the preparation of the infusion if you have been told so during the training.
- If necessary, program the pump according to the user manual and as you have been shown during the training by your healthcare provider.
Figure 2
3. Checking and opening the vials
- Allow products to reach room temperature (77°F / ≤ 25°C).
- Inspect each vial carefully:
Check that the labelled dose is correct and based on your prescription.
Check that the expiration date has not been passed.
Check the appearance of the solution (it should be clear and colorless to pale yellow or slightly opalescent).
Do not use the solution if it is cloudy or contains particles.
Make sure the protective cap is not broken or missing.
- Remove the protective cap.
- Disinfect the rubber stopper by using a sterile wipe and allow it to dry ( Figure 2 ).
Figure 3
4. Preparing and filling the syringe
- Open sterile syringe and needle or needleless transfer device.
- If a needleless transfer device is used, follow the instructions of the device manufacturer.
- If the transfer is done using needle and syringe, follow the instructions below:
Attach the needle to the syringe with a screw action.
Draw back on the plunger to fill the syringe with air. The amount of air should be roughly equal to the amount of solution needed from the vial.
Insert the needle into the center of the vial stopper and slowly turn the vial upside down. To avoid foaming, ensure that the tip of the needle is not in the solution; then inject air by pushing the plunger of the syringe.
Next, move the needle so that the tip is in the solution; then slowly draw up the desired volume of CUTAQUIG solution, making sure that the needle tip is always in the solution ( Figure 3 ).
Withdraw the needle from the vial.
This procedure might be repeated if you need multiple vials for the calculated dose.
When finished, remove the needle and dispose of it into the sharps bin.
Once finished, proceed to the next step.
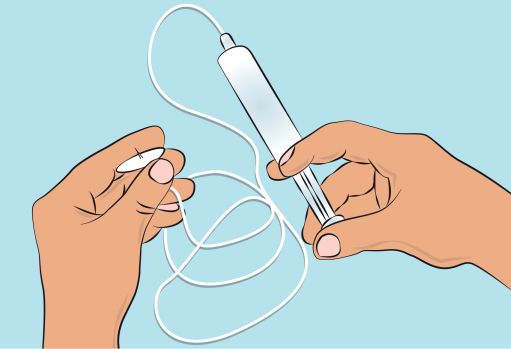
Figure 45. Preparing the infusion pump and tubing
- Follow the manufacturer’s instructions for preparing the infusion pump.
- To prime the administration tubing, attach the filled syringe to the infusion tubing and gently push the plunger to fill the tubing with CUTAQUIG, as has been shown to you during training ( Figure 4 ).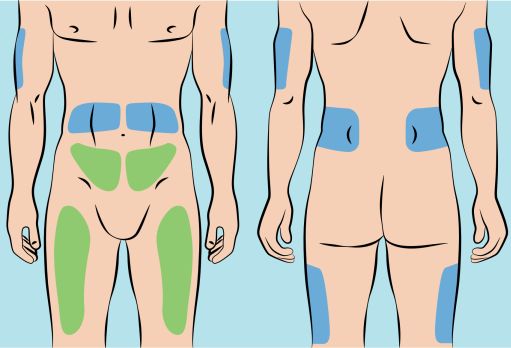
Figure 5
6. Preparing infusion site(s) and inserting the infusion needle(s)
- CUTAQUIG can be infused into the following areas: abdomen, thigh, upper arm, and/or upper leg/hip area ( Figure 5 ).
- The number and location of injection sites depends on the volume of the total dose.
- The infusion sites should be at least 2 inches apart. Do not use more than 6 infusion sites at the same time.
- Rotate sites between infusions.
- Avoid inserting the needle into scars, tattoos, stretch marks or any skin that has signs of infection (such as injured/inflamed/red skin area).
- Clean your skin at your selected infusion site(s) with an antiseptic skin wipe, starting at the center and working outward in a circular motion. Allow each site to dry before proceeding.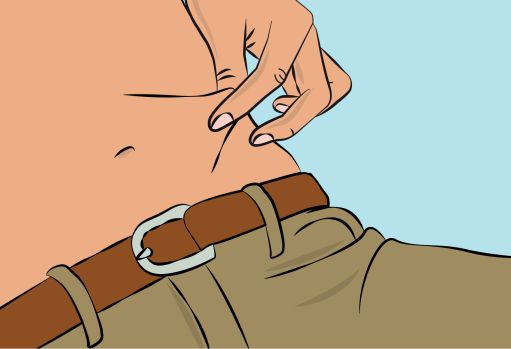
Figure 6- Pinch the skin between your thumb and forefinger around the injection site ( Figure 6 ). Carefully remove the needle cover and insert the needle into the skin ( Figure 7 ). The angle of the needle will depend on the type of infusion set being used.
- Put transparent dressing or sterile tape and gauze over the administration site to keep the needle in place during the infusion.
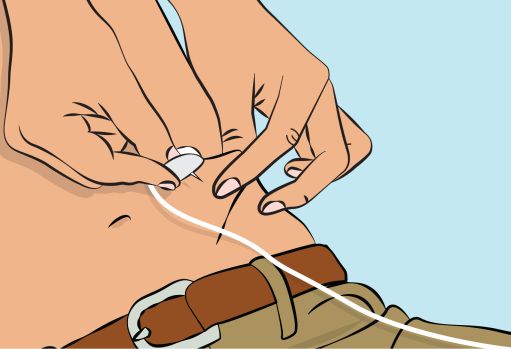
Figure 7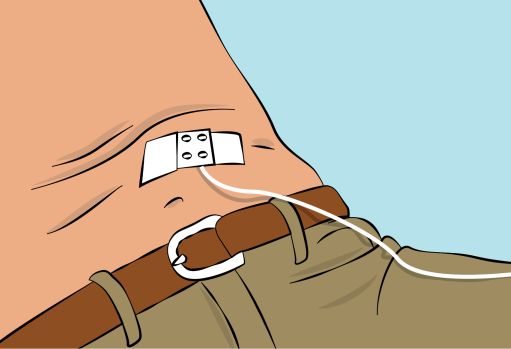
Figure 8
7. Checking the infusion
- Check needle placement by pulling back on the syringe plunger. There should not be any blood return in the tubing ( Figure 8 ).
- If blood return is seen, remove the needle and restart from step 6 with new tubing at a different location.
8. Starting the infusion
- Start the infusion. If an infusion pump is used for infusion, follow the manufacturer’s instructions.
9. Recording the infusion
- On each vial of CUTAQUIG, you will find a peel-off portion of the label with the batch number details. Stick this label in your patient’s treatment diary or infusion log book. Record details of the dose, date, time, infusion site location and any infections, side effects or other comments in connection with this infusion.
10. After infusion is complete
- Gently remove the dressing and the needle(s) and immediately place into the sharps bin.
- Press a small piece of gauze on the needle site and apply a dressing.
- Discard all used disposable supplies as well as any unused product and the empty vial(s) as recommended by your healthcare provider and according to local requirements.
- Tidy up and securely store all the reusable equipment (e.g., pump) until the next infusion.
If you encounter any problems or experience side effects during or after the infusion, contact your healthcare provider. When doing so, keep your treatment diary or log book with you to be able to give all necessary information.
You can also report side effects to the FDA at 1-800-FDA-1088 or online under www.fda.gov/medwatch.
For more product information on CUTAQUIG, please visit www.cutaquig.com
Manufactured by
Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Strasse 235
1100 Vienna, Austria
U.S. License No. 1646
Distributed by
Octapharma USA, Inc.
121 River Street, Suite 1201
Hoboken, NJ 07030
cutaquig ® is a registered trademark of Octapharma.
Issued 08 2019.
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
Immune Globulin Subcutaneous (Human) - hipp, 16.5%
Cutaquig
6 mL
NDC: 68982-810-01
Cutaquig
10 mL
NDC: 68982-810-02
Cutaquig
12 mL
NDC: 68982-810-03
Cutaquig
20 mL
NDC: 68982-810-04
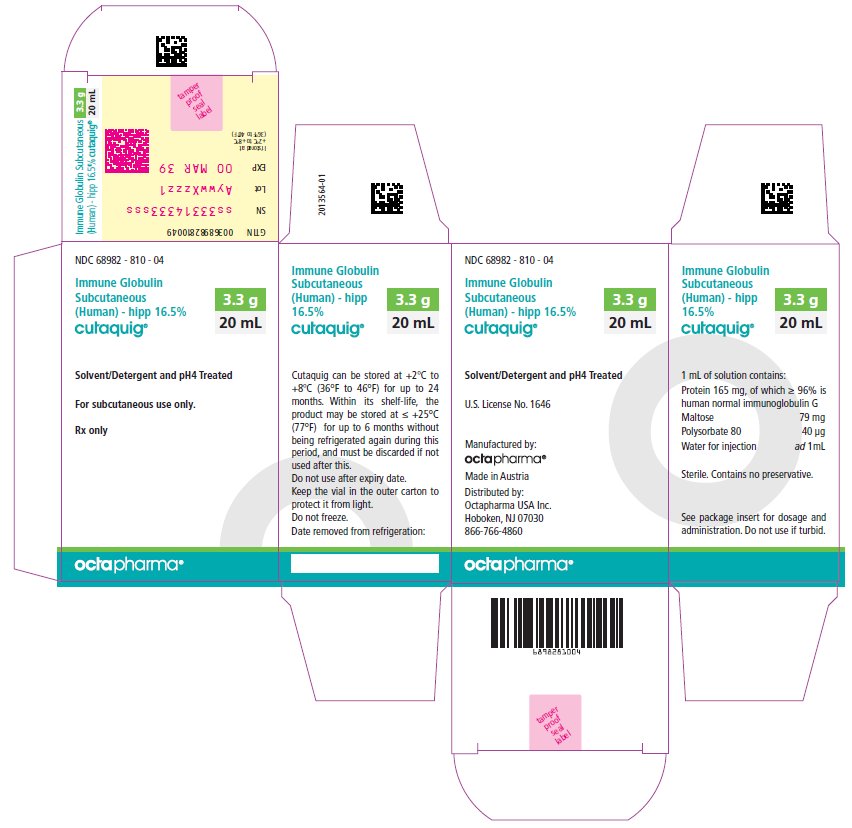
Cutaquig
24 mL
NDC: 68982-810-05
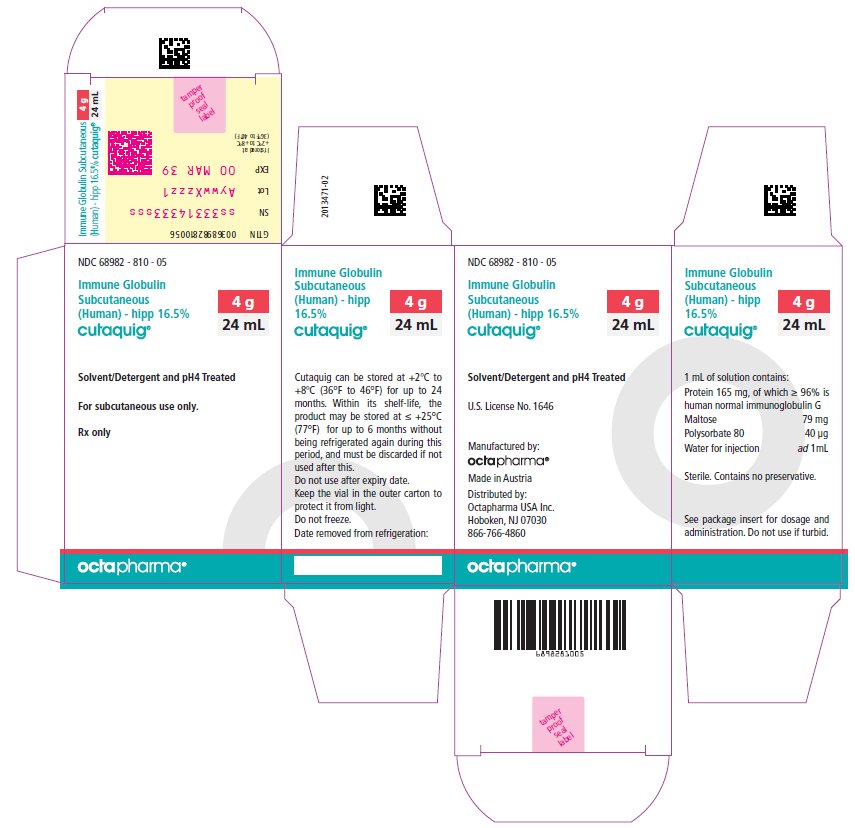
Cutaquig
48 mL
NDC: 68982-810-06
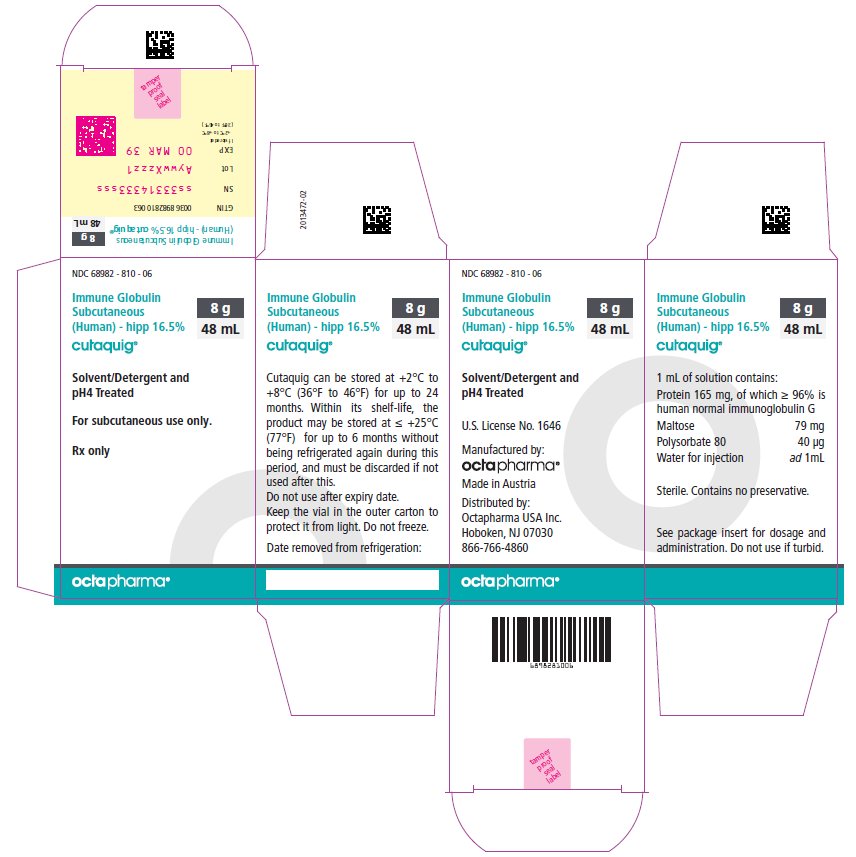
-
INGREDIENTS AND APPEARANCE
CUTAQUIG
immunoglobulin g solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68982-810 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN IMMUNOGLOBULIN G (UNII: 66Y330CJHS) (HUMAN IMMUNOGLOBULIN G - UNII:66Y330CJHS) HUMAN IMMUNOGLOBULIN G 165 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68982-810-01 1 in 1 CARTON 1 NDC: 68982-810-81 6 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 68982-810-02 1 in 1 CARTON 2 NDC: 68982-810-82 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 3 NDC: 68982-810-03 1 in 1 CARTON 3 NDC: 68982-810-83 12 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 4 NDC: 68982-810-04 1 in 1 CARTON 4 NDC: 68982-810-84 20 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 5 NDC: 68982-810-05 1 in 1 CARTON 5 NDC: 68982-810-85 24 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 6 NDC: 68982-810-06 1 in 1 CARTON 6 NDC: 68982-810-86 48 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125668 05/03/2019 Labeler - Octapharma USA Inc (606121163)
Trademark Results [Cutaquig]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CUTAQUIG 87225330 5880441 Live/Registered |
Octapharma AG 2016-11-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.