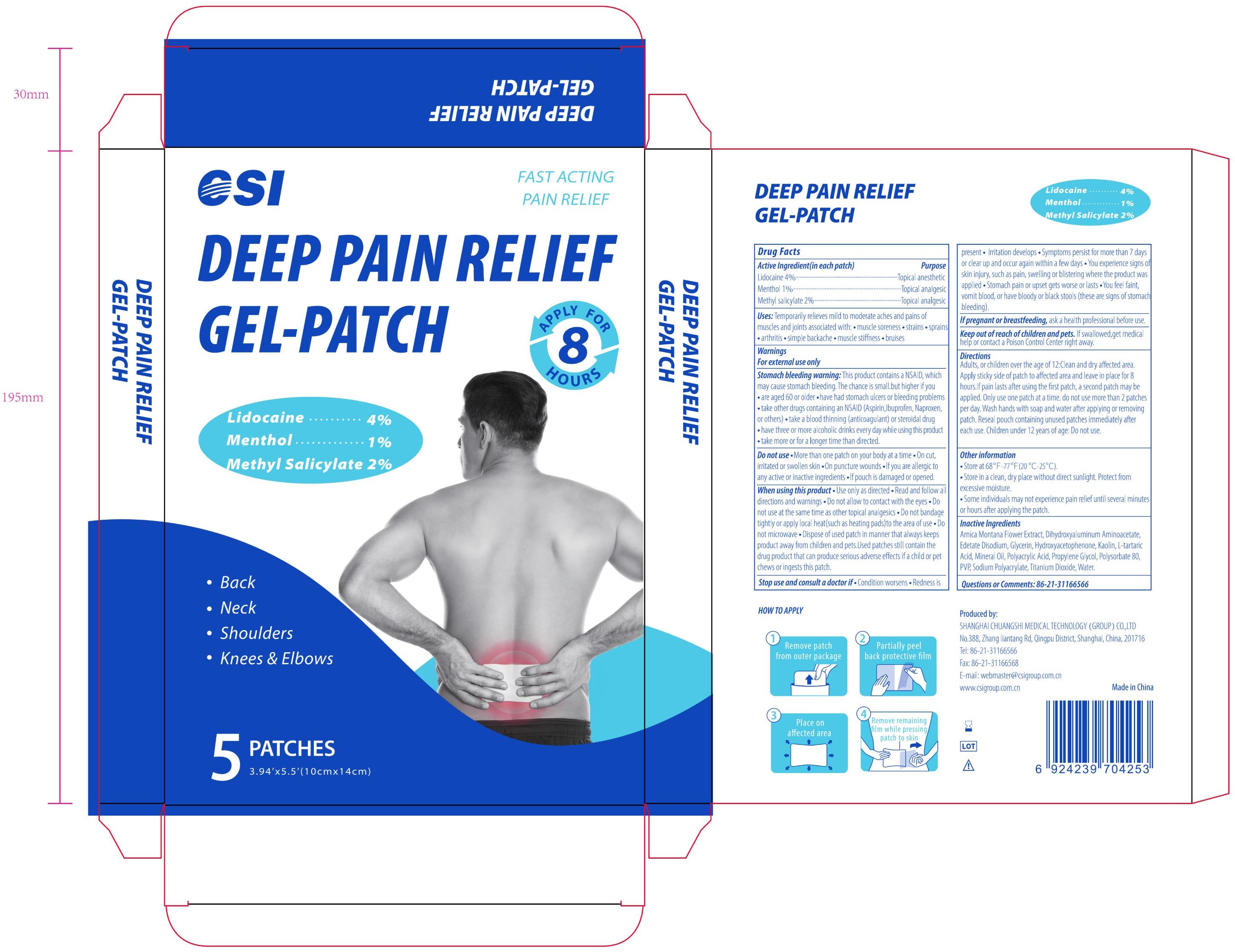
CSI, DEEP PAIN RELIEF, Lidocaine & Menthol & Methyl salicylate, 5 Patches
DEEP PAIN RELIEF by
Drug Labeling and Warnings
DEEP PAIN RELIEF by is a Otc medication manufactured, distributed, or labeled by Shanghai Chuangshi Medical Technology (Group) Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DEEP PAIN RELIEF- lidocaine, menthol, methyl salicylate patch
Shanghai Chuangshi Medical Technology (Group) Co., Ltd.
----------
CSI, DEEP PAIN RELIEF, Lidocaine & Menthol & Methyl salicylate, 5 Patches
Active Ingredient
Lidocaine 4% w/w ...... Purpose: Topical anesthetic
Menthol 1% w/w ...... Purpose: Topical analgesic
Methyl salicylate 2% w/w ...... Purpose: Topical analgesic
Purpose
Topical anesthetic (Lidocaine)
Topical analgesic (Menthol)
Topical analgesic (Methyl salicylate)
Uses
Temporarily relieves mild to moderate aches and pains of muscle and joints associated with: · muscle soreness · strains · sprains · arthritis · simple backache · muscle stiffness · bruises
Stomach bleeding warning
This product contains a NSAID, which may cause stomach bleeding. The chance is smaill but higher if you · are aged 60 or older · have had stomach ulcers or bleeding problems · take other drugs containing an NSAID (Aspirin, Ibuprofen, Naproxen, or others) · take a blood thinning (anticoagulant) or steroidal drug · have three or more alcoholic drinks every day while using this product · take more or for a longer time than directed.
Do not use
· More than one patch on your body at a time · On cut, irritated or swollen skin · On puncture wounds · If you are allergic to any active or inactive ingredients · If pouch is damaged or opened.
When using this product
· Use only as directed · Read and follow all directions and warnings · Do not allow to contact with the eyes · Do not use at the same time as other topical analgesics · Do not bandage tightly or apply local heat (such as heating pads) to the area of use · Do not microwave · Dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
Stop use and consult a doctor if
· Condition worsens · Redness is present · Irritation develops · Symptoms persist for more than 7 days or clear up and occur again within a few days · You experience signs of skin injury, such as pain, swelling or blistering where the product was applied · Stomach pain or upset gets worse or lasts · You feel faint, vomit blood, or have bloody or black stools (these are signs of stomach bleeding).
Keep out of reach of children and pets
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults, or children over the age of 12: Clean and dry affected area. Apply sticky side of patch to affected area and leave in place for 8 hours. If pain lasts after using the first patch, a second patch may be applied. Only use one patch at a time. Do not use more than 2 patches per day. Wash hands with soap and water after applying or removing patch. Reseal pouch containing unused patches immediately after each use.
Children under 12 years of age: Do not use.
Other information
· Store at 68℉-77℉ (20℃-25℃)
· Store in a clean, dry place without direct sunlight. Protect from excessive moisture.
· Some individuals may not experience pain relief until several minutes or hours after applying the patch.
| DEEP PAIN RELIEF
lidocaine, menthol, methyl salicylate patch |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) |
| Registrant - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shanghai Chuangshi Medical Technology (Group) Co., Ltd. | 546872672 | manufacture(73557-165) , label(73557-165) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.