Lidocaine 4% Anesthetic Cream
Lidocaine by
Drug Labeling and Warnings
Lidocaine by is a Otc medication manufactured, distributed, or labeled by County Line Pharmaceuticals, LLC, Ei LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LIDOCAINE- lidocaine cream
County Line Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
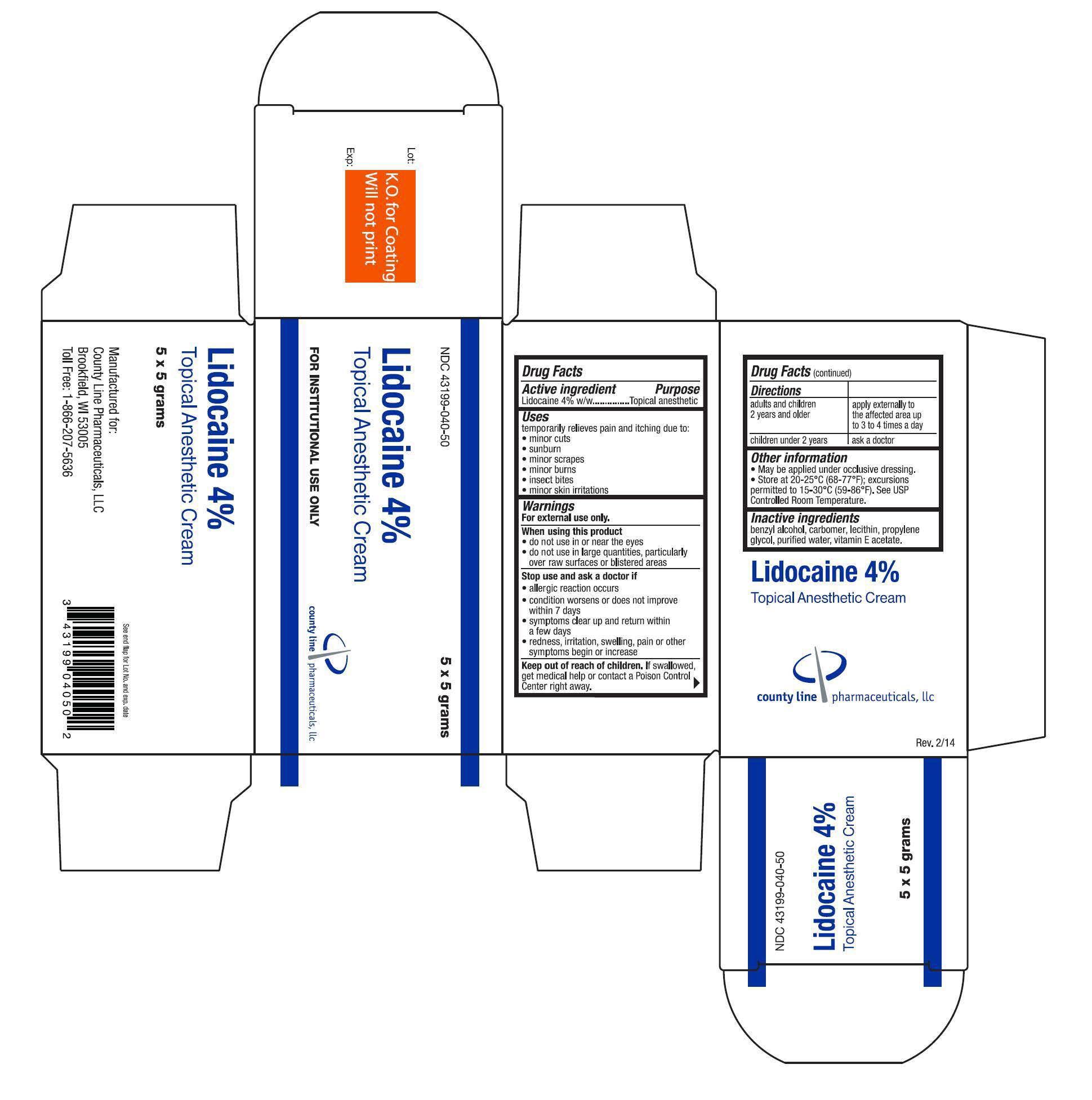
Lidocaine 4% Anesthetic Cream
Uses
temporarily relieves pain and itching due to:
- minor cuts
- sunburn
- minor scrapes
- minor burns
- insect bites
- minor skin irritations
Warnings
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
Directions
| adults and children 2 years and older | apply externally to the affected area up to 3 to 4 times a day |
| children under 2 years | ask a doctor |
Other information
- May be applied under occlusive dressing.
- Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
Inactive ingredients
benzyl alcohol, carbomer, lecithin, propylene glycol, purfied water, vitamin E acetate.
Lidocaine 4%
NDC: 43199-040-50
Lidocaine 4%
Topical Anesthetic Cream
FOR INSTITUTIONAL USE ONLY
5 x 5 grams
county line pharmaceuticals, llc
Lidocaine 4% - kit with Tegaderm Dressings
NDC: 43199-040-15
Lidocaine 4%
Topical Anesthetic Cream
FOR INSTITUTIONAL USE ONLY
Five 5 gram tubes + ten 3M TegadermTM Transparent Dressings
county line pharmaceuticals, llc

| LIDOCAINE
lidocaine cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - County Line Pharmaceuticals, LLC (015585278) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ei LLC | 105803274 | manufacture(43199-040) , analysis(43199-040) , label(43199-040) | |