83285-501 Dr.SEED HEARTLEAF B7 AQUA TREATMENT
Dr.SEED HEARTLEAF B7 AQUA TREATMENT by
Drug Labeling and Warnings
Dr.SEED HEARTLEAF B7 AQUA TREATMENT by is a Otc medication manufactured, distributed, or labeled by Saturday9 Co., Ltd., Yegreena Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
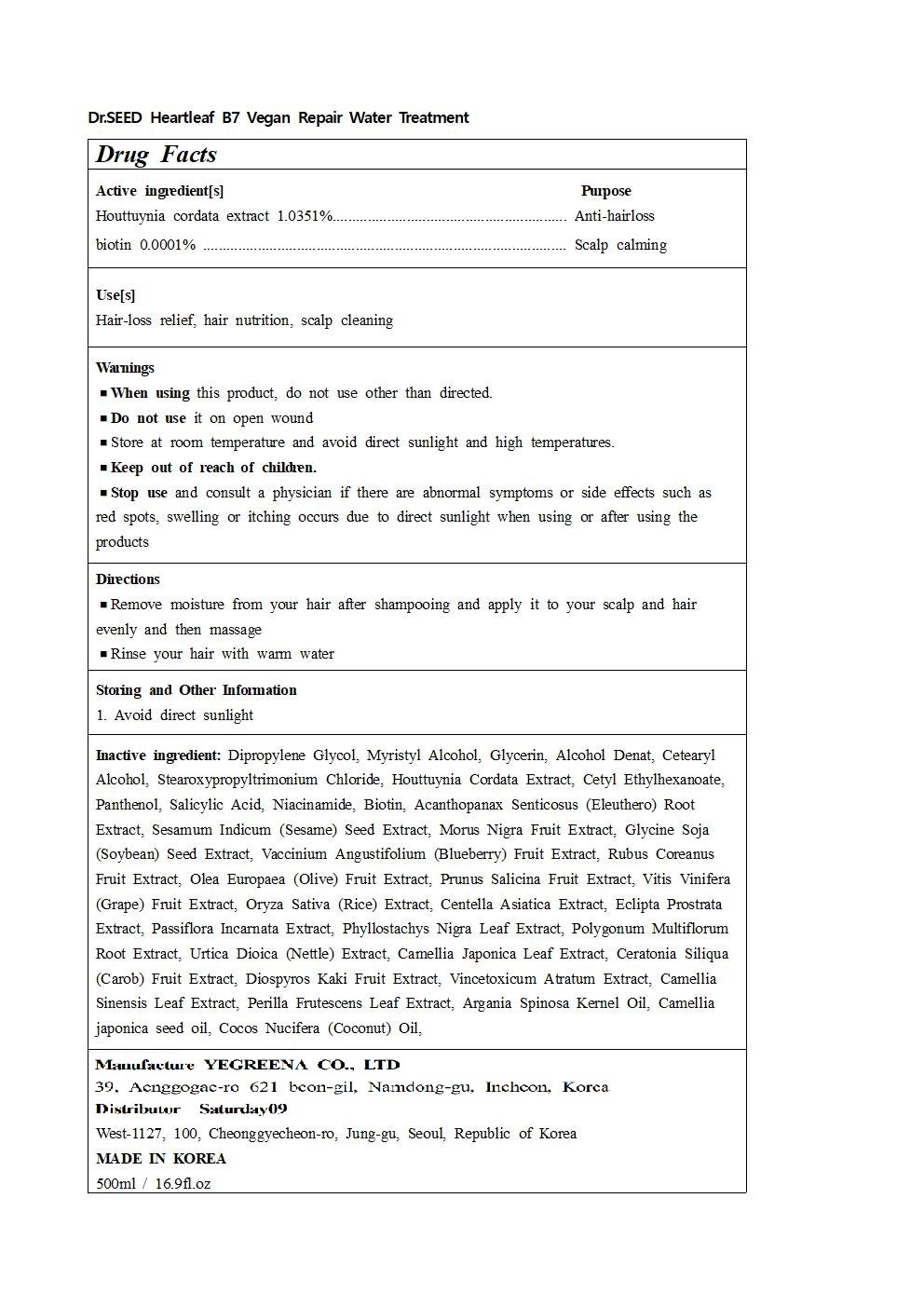
DR.SEED HEARTLEAF B7 AQUA TREATMENT- houttuynia cordata extract, biotin shampoo
Saturday9 Co., Ltd.
----------
83285-501 Dr.SEED HEARTLEAF B7 AQUA TREATMENT
Warnings
Stop use and consult a physician if there are abnormal symptoms or side effects such as red spots, swelling or itching occurs due to direct sunlight when using or after using the products
Do not use it on open wound
When using the product, wash the eyes immediately if it gets into the eyes
Keep out of reach of Children
Warnings
Stop use and consult a physician if there are abnormal symptoms or side effects such as red spots, swelling or itching occurs due to direct sunlight when using or after using the products
Directions
Remove moisture from your hair after shampooing and apply it to your scalp and hair evenly and then massage
Rinse your hair with warm water
Inactive Ingredients
Dipropylene Glycol, Myristyl Alcohol, Glycerin, Alcohol Denat, Cetearyl Alcohol, Stearoxypropyltrimonium Chloride
Houttuynia Cordata Extract, Cetyl Ethylhexanoate, Panthenol, Salicylic Acid, Niacinamide, Biotin
Acanthopanax Senticosus (Eleuthero) Root Extract, Sesamum Indicum (Sesame) Seed Extract, Morus Nigra Fruit Extract
Glycine Soja (Soybean) Seed Extract, Vaccinium Angustifolium (Blueberry) Fruit Extract, Rubus Coreanus Fruit Extract
Olea Europaea (Olive) Fruit Extract, Prunus Salicina Fruit Extract, Vitis Vinifera (Grape) Fruit Extract
Oryza Sativa (Rice) Extract, Centella Asiatica Extract, Eclipta Prostrata Extract, Passiflora Incarnata Extract
Phyllostachys Nigra Leaf Extract, Polygonum Multiflorum Root Extract, Urtica Dioica (Nettle) Extract
Camellia Japonica Leaf Extract, Ceratonia Siliqua (Carob) Fruit Extract, Diospyros Kaki Fruit Extract
Vincetoxicum Atratum Extract, Camellia Sinensis Leaf Extract, Perilla Frutescens Leaf Extract
Argania Spinosa Kernel Oil, Camellia japonica seed oil, Cocos Nucifera (Coconut) Oil
Hippophae Rhamnoides Oil, Limnanthes Alba (Meadowfoam) Seed Oil, Moringa Oleifera Seed Oil
Olea Europaea (Olive) Fruit Oil, Persea Gratissima (Avocado) Oil, Prunus Armeniaca (Apricot) Kernel Oil
Ricinus Communis (Castor) Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Butylene Glycol
1,2-Hexanediol, Isopropyl Myristate, Cetyl Alcohol, Stearyl Alcohol, Isoamyl Laurate, Isopropyl Palmitate
Ethylhexylglycerin, Water, Fragrance
| DR.SEED HEARTLEAF B7 AQUA TREATMENT
houttuynia cordata extract, biotin shampoo |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Saturday9 Co., Ltd. (694918874) |
| Registrant - Saturday9 Co., Ltd. (694918874) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Yegreena Co., Ltd. | 695951904 | manufacture(83285-501) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.