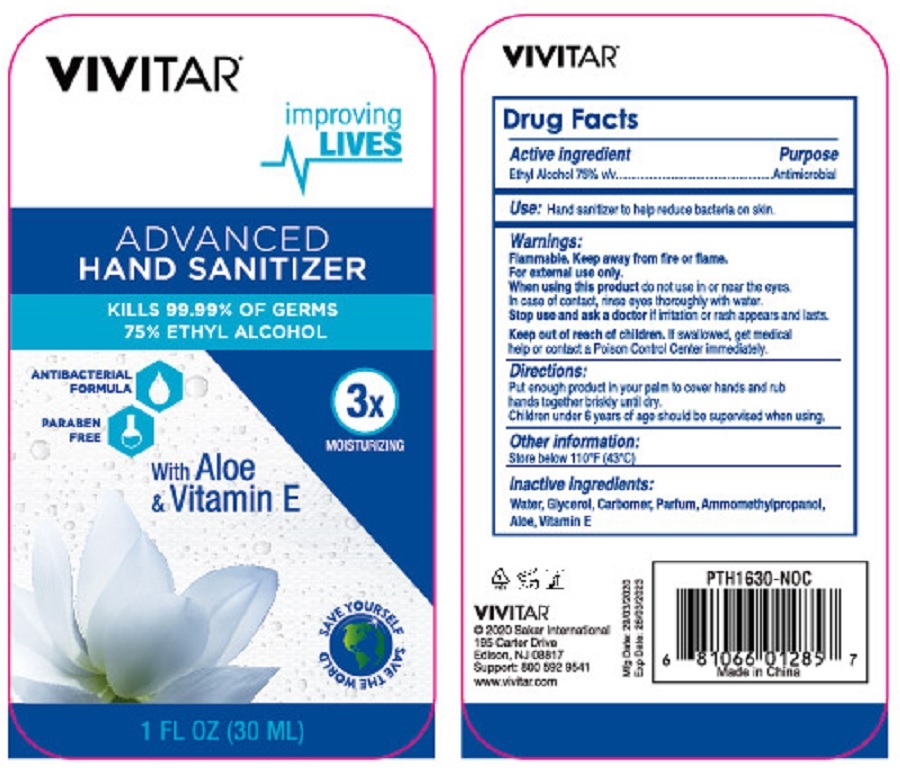
VIVITAR ADVANCED HAND SANITIZER
Vivitar Advanced Hand Sanitizer by
Drug Labeling and Warnings
Vivitar Advanced Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Sakar International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VIVITAR ADVANCED HAND SANITIZER- ethyl alcohol gel
Sakar International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
VIVITAR ADVANCED HAND SANITIZER
Warnings:
Flammable. Keep away from fire or flame.
For external use only.
When using this product do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation and rash appears and lasts.
Directions:
Put enough product in your palm to cover hands and rub hands together briskly until dry.
Children under 6 years of age should be supervised when using.
| VIVITAR ADVANCED HAND SANITIZER
ethyl alcohol gel |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Sakar International, Inc. (086413028) |