FLUOCINOLONE ACETONIDE OIL- fluocinolone acetonide oil
Fluocinolone Acetonide by
Drug Labeling and Warnings
Fluocinolone Acetonide by is a Prescription medication manufactured, distributed, or labeled by Rising Pharmaceuticals, Inc., Lyne Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Fluocinolone Acetonide Topical Oil, 0.01% (Body Oil) safely and effectively. See full prescribing information for Fluocinolone Acetonide Topical Oil, 0.01%.
Fluocinolone Acetonide Topical Oil, 0.01% (Body Oil)
For topical use only
Initial U.S. Approval: 1988
NDC: 64980-331-04
For Topical Use Only - Not for Oral, Ophthalmic, or Intravaginal Use
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Fluocinolone Acetonide Topical Oil, 0.01% (Body Oil) is supplied in bottles containing 4 fluid ounces. (3)
CONTRAINDICATIONS
None (4).
WARNINGS AND PRECAUTIONS
- Topical corticosteroids can produce reversible HPA axis suppression, Cushing's syndrome, hyperglycemia, and glucosuria. (5.1)
- Systemic absorption may require evaluation for hypothalamic-pituitary-adrenal (HPA) axis suppression. (5.1)
- Modify use should HPA axis suppression develop. (5.1)
- Potent corticosteroids use on large areas, prolonged use or occlusive use may increase systemic absorption. (5.1)
- Local adverse reactions may include atrophy, striae, irritation, acneiform eruptions, hypopigmentation, and allergic contact dermatitis and may be more likely with occlusive use or more potent corticosteroids. (5.2, 5.3, 6.1)
- Children may be more susceptible to systemic toxicity from equivalent doses. (5.1, 8.4)
ADVERSE REACTIONS
The most common adverse reactions (≥5%) were cough (20%), rhinorrhea (13%), pyrexia (10%), telangiectasia (7%), nasopharyngitis (7%), and hypopigmentation (7%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharmaceuticals, Inc. at 1-866-562-4597 or FDA at 1-800-FDA-1088 or www.FDA.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adult Patients with Atopic Dermatitis
1.2 Pediatric Patients with Atopic Dermatitis
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Adult Patients with Atopic Dermatitis
2.2 Pediatric Patients with Atopic Dermatitis
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal Axis Suppression
5.2 Local Adverse Reactions with Topical Corticosteroids
5.3 Allergic Contact Dermatitis with Topical Corticosteroids
5.4 Concomitant Skin Infections
5.5 Use in Peanut-Sensitive Individuals
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience: Evaluation of Facial Use in Pediatric Subjects
6.2 Clinical Studies Experience: Evaluation in Pediatric Subjects 3 months to 2 years old
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, mutagenesis, impairment of fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Instructions
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Adult Patients with Atopic Dermatitis
Fluocinolone acetonide topical oil, 0.01% is indicated for the topical treatment of atopic dermatitis in adult patients.
1.2 Pediatric Patients with Atopic Dermatitis
Fluocinolone acetonide topical oil, 0.01% is indicated for the topical treatment of moderate to severe atopic dermatitis in pediatric patients, 3 months and older for up to 4 weeks. Safety and effectiveness in pediatric patients younger than 3 months of age have not been established.
1.3 Limitations of Use
Apply the least amount of fluocinolone acetonide topical oil, 0.01% needed to cover the affected areas. As with other corticosteroids, fluocinolone acetonide topical oil, 0.01% should be discontinued when control of disease is achieved. Contact the physician if no improvement is seen within 2 weeks. Fluocinolone acetonide topical oil, 0.01% should not be applied to the diaper area; diapers or plastic pants may constitute occlusive use.
Fluocinolone acetonide topical oil, 0.01% should not be used on the face, axillae, or groin unless directed by the physician. Application to intertriginous areas should be avoided due to the increased risk of local adverse reactions.[see Adverse Reactions (6) and Use in Specific Populations (8.4)]. -
2 DOSAGE AND ADMINISTRATION
Fluocinolone acetonide topical oil, 0.01% is not for oral, ophthalmic, or intravaginal use. The dosing of fluocinolone acetonide topical oil, 0.01% is different for adult and pediatric patients.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal Axis Suppression
Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency. Cushing’s syndrome, hyperglycemia, and glucosuria can also be produced by systemic absorption of topical corticosteroids.
Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. The ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression.
If HPA axis suppression is documented, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent corticosteroid.
Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids.
Conditions which increase systemic absorption include the use of more potent corticosteroids, use over large surface areas, use over prolonged periods, and use of occlusive dressings. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroids.
Children may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios. [See Use in Specific Populations (8.4)]
5.2 Local Adverse Reactions with Topical Corticosteroids
Local adverse reactions may occur with use of topical corticosteroids and may be more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids. Some local adverse reactions may be irreversible. Reactions may include atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. [See Adverse Reactions (6.1)]
5.3 Allergic Contact Dermatitis with Topical Corticosteroids
Allergic contact dermatitis to any component of topical corticosteroids is usually diagnosed by a failure to heal rather than a clinical exacerbation. Clinical diagnosis of allergic contact dermatitis can be confirmed by patch testing.
5.4 Concomitant Skin Infections
Concomitant skin infections should be treated with an appropriate antimicrobial agent. If the infection persists unchanged, fluocinolone acetonide topical oil, 0.01% should be discontinued until the infection has been adequately treated.
5.5 Use in Peanut-Sensitive Individuals
Physicians should use caution in prescribing fluocinolone acetonide topical oil, 0.01% for peanut-sensitive individuals. [See Description (11)]
Should signs of hypersensitivity present (wheal and flare reactions, pruritus, or other manifestations), or should disease exacerbations occur, fluocinolone acetonide topical oil, 0.01% should be discontinued immediately and appropriate therapy instituted.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Studies Experience: Evaluation of Facial Use in Pediatric Subjects
An open-label study was conducted in 58 children with moderate to severe atopic dermatitis (2 to 12 years old) to evaluate the safety of fluocinolone acetonide topical oil, 0.01% when applied to the face twice daily for 4 weeks. The following adverse reactions were reported:
Incidence of Adverse Reactions (%), N=58 Adverse Reaction (AR)* # of subjects (%) Day 14 Day 28† Day 56‡ - * The number of individual adverse reactions reported does not necessarily reflect the number of individual subjects, since one subject could have multiple reporting of an adverse reaction.
- † End of Treatment
- ‡ Four Weeks Post Treatment
Any AE 15 (26) 6 (10) 7 (12) 7 (12) Telangiectasia 5 (9) 3 (5) 4 (7) 2 (4) Erythema 3 (5) 3 (5) Itching 3 (5) 3 (5) Irritation 3 (5) 3 (5) Burning 3 (5) 3 (5) Hypopigmentation 2 (4) 2 (4) Shiny skin 1 (2) 1 (2) Secondary atopic dermatitis 1 (2) 1 (2) Papules and pustules 1 (2) 1 (2) Keratosis pilaris 1 (2) 1 (2) Folliculitis 1 (2) 1 (2) Facial herpes simplex 1 (2) 1 (2) Acneiform eruption 1 (2) 1 (2) Ear infection 1 (2) 1 (2) 6.2 Clinical Studies Experience: Evaluation in Pediatric Subjects 3 months to 2 years old
An open-label safety study was conducted in 29 children to assess the HPA axis by ACTH stimulation testing following use of fluocinolone acetonide topical oil, 0.01% twice daily for 4 weeks. The following adverse reactions were reported in the study [See Use in Specific Populations (8.4)]:
Adverse Reactions (%), N=30* Adverse Reaction # of subjects (%) - * Includes one subject who withdrew at Week 2
Diarrhea 1 (3) Vomiting 1 (3) Pyrexia 3 (10) Abscess 1 (3) Molluscum 1 (3) Nasopharyngitis 2 (7) URI 1 (3) Otitis media 1 (3) Cough 6 (20) Rhinorrhea 4 (13) Atopic dermatitis 1 (3) Eczema 1 (3) Hyperpigmentation 1 (3) Hypopigmentation 2 (7) Rash 1 (3) -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
There are no adequate and well-controlled studies in pregnant women on teratogenic effects from fluocinolone acetonide topical oil, 0.01%. Therefore, fluocinolone acetonide topical oil, 0.01% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when fluocinolone acetonide topical oil, 0.01% is administered to a nursing woman.
8.4 Pediatric Use
8.4.1 Systemic Adverse Reactions in Pediatric Patients HPA axis suppression, Cushing’s syndrome, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include linear growth retardation, delayed weight gain, low plasma cortisol levels, and subnormal response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Because of a higher ratio of skin surface area to body mass, children are at a greater risk for systemic adverse reactions than are adults when treated with topical corticosteroids. [See Warnings and Precautions (5.1)]
8.4.2 Evaluation in Peanut-Sensitive Pediatric Subjects A clinical study was conducted to assess the safety of fluocinolone acetonide topical oil, 0.01%, which contains refined peanut oil, on subjects with known peanut allergies. The study enrolled 13 subjects with atopic dermatitis, 6 to 17 years of age. Of the 13 subjects, 9 were Radioallergosorbent Test (RAST) positive to peanuts and 4 had no peanut sensitivity (controls). The study evaluated the subjects’ responses to both prick test and patch test utilizing peanut oil NF, fluocinolone acetonide topical oil, 0.01% and histamine/saline controls. Subjects were also treated with fluocinolone acetonide topical oil, 0.01% twice daily for 7 days. Prick test and patch test results for all 13 patients were negative to fluocinolone acetonide topical oil, 0.01% and the refined peanut oil. One of the 9 peanut-sensitive patients experienced an exacerbation of atopic dermatitis after 5 days of fluocinolone acetonide topical oil, 0.01%. The bulk peanut oil NF, used in fluocinolone acetonide topical oil, 0.01% is heated between 232˚C – 246˚C (450˚F – 475˚F) for at least 15 minutes, which should provide for adequate decomposition of allergenic proteins. [See Description (11)]
8.4.3 Evaluation in Pediatric Subjects 2 to 6 years old Open-label safety studies were conducted on 33 children (20 subjects ages 2 to 6 years, 13 subjects ages 7 to 12 years) with moderate to severe stable atopic dermatitis. Subjects were treated with fluocinolone acetonide topical oil, 0.01% twice daily for 4 weeks. Baseline body surface area involvement was 50% to 75% in 15 subjects and greater than 75% in 18 subjects. Morning pre-stimulation cortisol and post-ACTH stimulation cortisol levels were obtained in each subject at the beginning of the trial and at the end of 4 weeks of treatment. At the end of treatment, 4 out of 18 subjects aged 2 to 5 years showed low pre-stimulation cortisol levels (3.2 to 6.6 µg/dL; normal: cortisol > 7 µg/dL) but all had normal responses to 0.25 mg of ACTH stimulation (cortisol > 18 µg/dL).
8.4.4 Evaluation in Pediatric Subjects 3 months to 2 years old An open-label safety study was conducted in 29 children (7 subjects ages 3 to 6 months, 7 subjects ages > 6 to 12 months and 15 subjects ages > 12 months to 2 years of age) to assess the HPA axis by ACTH stimulation testing following use of fluocinolone acetonide topical oil, 0.01% twice daily for 4 weeks. All subjects had moderate to severe atopic dermatitis with disease involvement on at least 20% body surface area. Baseline body surface area involvement was 50% to 75% in 11 subjects and greater than 75% in 7 subjects. Morning pre-stimulation and post-ACTH stimulation cortisol levels were obtained in each subject at the beginning of the trial and at the end of 4 weeks of treatment. All subjects had normal responses to 0.125 mg of ACTH stimulation (cortisol >18 µg/dL).
-
10 OVERDOSAGE
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects, including under conditions of normal use. [See Warnings and Precautions (5.1) and Use in Specific Populations (8.4)].
-
11 DESCRIPTION
Fluocinolone Acetonide Topical Oil, 0.01% (Body Oil) contains fluocinolone acetonide [(6α, 11β, 16α)-6,9-difluoro-11,21-dihydroxy-16,17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione, cyclic 16,17 acetal with acetone], a synthetic corticosteroid for topical dermatologic use. Chemically, fluocinolone acetonide is C24H30F2O6. It has the following structural formula:
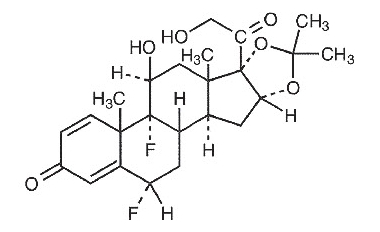
Fluocinolone acetonide in fluocinolone acetonide topical oil, 0.01% has a molecular weight of 452.50. It is a white crystalline powder that is odorless, stable in light, and melts at 270°C with decomposition; soluble in alcohol, acetone and methanol; slightly soluble in chloroform; insoluble in water.
Each gram of fluocinolone acetonide topical oil, 0.01% contains approximately 0.11 mg of fluocinolone acetonide in a blend of oils, which contains isopropyl alcohol, isopropyl myristate, light mineral oil, oleth-2 and refined peanut oil NF.
Fluocinolone acetonide topical oil, 0.01% is formulated with 48% refined peanut oil NF. Physicians should use caution in prescribing fluocinolone acetonide topical oil, 0.01% for peanut-sensitive individuals.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Like other topical corticosteroids, fluocinolone acetonide has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
12.3 Pharmacokinetics
Topical corticosteroids can be absorbed from intact healthy skin. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the product formulation and the integrity of the epidermal barrier. Occlusion, inflammation and/or other disease processes in the skin may increase percutaneous absorption. The use of pharmacodynamic endpoints for assessing the systemic exposure of topical corticosteroids may be necessary due to the fact that circulating levels are often below the level of detection. Once absorbed through the skin, topical corticosteroids are metabolized primarily in the liver, and are then excreted by the kidneys. Some corticosteroids and their metabolites are also excreted in the bile.
Fluocinolone acetonide topical oil, 0.01% is in the low to medium range of potency as compared with other topical corticosteroids in vasoconstrictor studies.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, mutagenesis, impairment of fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of fluocinolone acetonide topical oil, 0.01%. Studies have not been performed to evaluate the mutagenic potential of fluocinolone acetonide, the active ingredient in fluocinolone acetonide topical oil, 0.01%. Some corticosteroids have been found to be genotoxic in various genotoxicity tests (i.e. the in vitro human peripheral blood lymphocyte chromosome aberration assay with metabolic activation, the in vivo mouse bone marrow micronucleus assay, the Chinese hamster micronucleus test, and the in vitro mouse lymphoma gene mutation assay).
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
17.1 Instructions
- Fluocinolone acetonide topical oil, 0.01% should be used as directed by the physician. It is for external use only. Avoid contact with the eyes. In case of contact, wash eyes liberally with water.
- Fluocinolone acetonide topical oil, 0.01% should not be used for any disorder other than that for which it was prescribed.
- Patients should report any worsening of their skin condition to their physician promptly.
- Fluocinolone acetonide topical oil, 0.01% should not be applied under occlusion unless directed by the physician. Fluocinolone acetonide topical oil, 0.01% should not be applied to the diaper area as diapers or plastic pants may constitute occlusive use.
- Fluocinolone acetonide topical oil, 0.01% should not be used on the face, axillae, or groin unless directed by the physician.
- As with other corticosteroids, therapy should be discontinued when control of disease is achieved. Contact the physician if no improvement is seen within 2 weeks.
- Do not use other corticosteroid-containing products while using fluocinolone acetonide topical oil, 0.01% without first consulting your physician.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
———PRINCIPAL DISPLAY PANEL———
Rising®
NDC: 64980-331-04
Fluocinolone
Acetonide
Topical Oil,
0.01%
(Body Oil)
FOR TOPICAL USE ONLY
NOT FOR ORAL,
OPHTHALMIC,
or INTRAVAGINAL USE
SHAKE WELL BEFORE USE
Net Contents
118.28 mL
(4 fl. oz.) Rx only

-
INGREDIENTS AND APPEARANCE
FLUOCINOLONE ACETONIDE OIL
fluocinolone acetonide oilProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64980-331 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOCINOLONE ACETONIDE (UNII: 0CD5FD6S2M) (FLUOCINOLONE ACETONIDE - UNII:0CD5FD6S2M) FLUOCINOLONE ACETONIDE 0.11 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LIGHT MINERAL OIL (UNII: N6K5787QVP) OLETH-2 (UNII: 7L6R1SQ6M0) PEANUT OIL (UNII: 5TL50QU0W4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64980-331-04 118.28 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/18/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203377 07/18/2016 Labeler - Rising Pharmaceuticals, Inc. (041241766) Establishment Name Address ID/FEI Business Operations Lyne Laboratories, Inc. 053510459 manufacture(64980-331)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.