Eptifibatide by Amneal Pharmaceuticals LLC / Gland Pharma Limited EPTIFIBATIDE injection
Eptifibatide by
Drug Labeling and Warnings
Eptifibatide by is a Prescription medication manufactured, distributed, or labeled by Amneal Pharmaceuticals LLC, Gland Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EPTIFIBATIDE INJECTION safely and effectively. See full prescribing information for EPTIFIBATIDE INJECTION .
EPTIFIBATIDE injection, for intravenous use
Initial U.S. Approval: 1998INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Bleeding diathesis or bleeding within the previous 30 days (4)
- Severe uncontrolled hypertension (4)
- Major surgery within the preceding 6 weeks (4)
- Stroke within 30 days or any history of hemorrhagic stroke (4)
- Coadministration of another parenteral GP IIb/IIIa inhibitor (4)
- Dependency on renal dialysis (4)
- Known hypersensitivity to any component of the product (4)
WARNINGS AND PRECAUTIONS
- Eptifibatide can cause serious bleeding. If bleeding cannot be controlled, discontinue eptifibatide immediately. Minimize vascular and other traumas. If heparin is given concomitantly, monitor aPTT or ACT. (5.1)
- Thrombocytopenia: Discontinue eptifibatide and heparin. Monitor and treat condition appropriately. (5.2)
ADVERSE REACTIONS
Bleeding and hypotension are the most commonly reported adverse reactions. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Biosciences at 1-855-266-3251 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Coadministration of antiplatelet agents, thrombolytics, heparin, aspirin and chronic NSAID use increases the risk of bleeding. Avoid concomitant use with other glycoprotein (GP) IIb/IIIa inhibitors. (7.1)
USE IN SPECIFIC POPULATIONS
- Geriatric Use: Risk of bleeding increases with age. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Acute Coronary Syndrome (ACS)
1.2 Percutaneous Coronary Intervention (PCI)
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Acute Coronary Syndrome (ACS)
2.2 Dosage in Percutaneous Coronary Intervention (PCI)
2.3 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bleeding
5.2 Thrombocytopenia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Use of Thrombolytics, Anticoagulants and Other Antiplatelet Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Non-ST-Segment Elevation Acute Coronary Syndrome
14.2 Percutaneous Coronary Intervention (PCI)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Acute Coronary Syndrome (ACS)
Eptifibatide injection is indicated to decrease the rate of a combined endpoint of death or new myocardial infarction (MI) in patients with ACS (unstable angina [UA]/non-ST-elevation myocardial infarction [NSTEMI]), including patients who are to be managed medically and those undergoing percutaneous coronary intervention (PCI).
1.2 Percutaneous Coronary Intervention (PCI)
Eptifibatide injection is indicated to decrease the rate of a combined endpoint of death, new MI, or need for urgent intervention in patients undergoing PCI, including those undergoing intracoronary stenting [see Clinical Studies (14.1,14.2)].
-
2 DOSAGE AND ADMINISTRATION
Before infusion of eptifibatide injection, the following laboratory tests should be performed to identify pre-existing hemostatic abnormalities: hematocrit or hemoglobin, platelet count, serum creatinine and PT/aPTT. In patients undergoing PCI, the activated clotting time (ACT) should also be measured.
The activated partial thromboplastin time (aPTT) should be maintained between 50 and 70 seconds unless PCI is to be performed. In patients treated with heparin, bleeding can be minimized by close monitoring of the aPTT and ACT.
2.1 Dosage in Acute Coronary Syndrome (ACS)
Indication Normal Renal Function Creatinine Clearance <50 mL/min Patients with ACS 180 mcg/kg intravenous (IV) bolus as soon as possible after diagnosis, followed by continuous infusion of 2 mcg/kg/min 180 mcg/kg IV bolus as soon as possible after diagnosis, followed by continuous infusion of 1 mcg/kg/min - Infusion should continue until hospital discharge or initiation of coronary artery bypass graft surgery (CABG), up to 72 hours
- If a patient is to undergo PCI, the infusion should be continued until hospital discharge or for up to 18 to 24 hours after the procedure, whichever comes first, allowing for up to 96 hours of therapy
- Aspirin, 160 to 325 mg, should be given daily
Eptifibatide injection should be given concomitantly with heparin dosed to achieve the following parameters:
During Medical Management: Target aPTT 50 to 70 seconds
- If weight greater than or equal to 70 kg, 5000-unit bolus followed by infusion of 1000 units/h.
- If weight less than 70 kg, 60-units/kg bolus followed by infusion of 12 units/kg/h.
During PCI: Target ACT 200 to 300 seconds
- If heparin is initiated prior to PCI, additional boluses during PCI to maintain an ACT target of 200 to 300 seconds.
- Heparin infusion after the PCI is discouraged.
2.2 Dosage in Percutaneous Coronary Intervention (PCI)
Indication Normal Renal Funtion Creatinine Clearance <50 mL/min Patients with PCI 180 mcg/kg IV bolus immediately before PCI followed by continuous infusion of 2 mcg/kg/min and a second bolus of 180 mcg/kg (given 10 minutes after the first bolus) 180 mcg/kg IV bolus immediately before PCI followed by continuous infusion of 1 mcg/kg/min and a second bolus of 180 mcg/kg (given 10 minutes after the first bolus) - Infusion should be continued until hospital discharge, or for up to 18 to 24 hours, whichever comes first. A minimum of 12 hours of infusion is recommended.
- In patients who undergo CABG surgery, eptifibatide injection infusion should be discontinued prior to surgery.
- Aspirin, 160 to 325 mg, should be given 1 to 24 hours prior to PCI and daily thereafter
- Eptifibatide injection should be given concomitantly with heparin to achieve a target ACT of 200 to 300 seconds. Administer 60-units/kg bolus initially in patients not treated with heparin within 6 hours prior to PCI.
- Additional boluses during PCI to maintain ACT within target.
- Heparin infusion after the PCI is strongly discouraged.
Patients requiring thrombolytic therapy should discontinue eptifibatide injection.
2.3 Important Administration Instructions
- Inspect eptifibatide injection for particulate matter and discoloration prior to administration, whenever solution and container permit.
- May administer eptifibatide injection in the same intravenous line as alteplase, atropine, dobutamine, heparin, lidocaine, meperidine, metoprolol, midazolam, morphine, nitroglycerin, or verapamil. Do not administer eptifibatide injection through the same intravenous line as furosemide.
- May administer eptifibatide injection in the same IV line with 0.9% NaCl or 0.9% NaCl/5% dextrose. With either vehicle, the infusion may also contain up to 60 mEq/L of potassium chloride.
- Withdraw the bolus dose(s) of eptifibatide injection from the 10-mL vial into a syringe. Administer the bolus dose(s) by IV push.
- Immediately following the bolus dose administration, initiate a continuous infusion of eptifibatide injection. When using an intravenous infusion pump, administer eptifibatide injection undiluted directly from the 100-mL vial. Spike the 100-mL vial with a vented infusion set. Center the spike within the circle on the stopper top.
- Discard any unused portion left in the vial.
Administer eptifibatide injection by volume according to patient weight (see Table 1).
Table 1: Eptifibatide Injection Dosing Charts by Weight Patient Weight 180-mcg/kg
Bolus Volume2-mcg/kg/min
Infusion Volume
(CrCl ≥50 mL/min)1-mcg/kg/min
Infusion Volume
(CrCl <50 mL/min)(kg) (lb) (from
2-mg/mL vial)(from 2-mg/mL
100-mL vial)(from 0.75-mg/mL
100-mL vial)(from 2-mg/mL
100-mL vial)(from 0.75-mg/mL
100-mL vial)37 to 41 81 to 91 3.4 mL 2 mL/h 6 mL/h 1 mL/h 3 mL/h 42 to 46 92 to 102 4 mL 2.5 mL/h 7 mL/h 1.3 mL/h 3.5 mL/h 47 to 53 103 to 117 4.5 mL 3 mL/h 8 mL/h 1.5 mL/h 4 mL/h 54 to 59 118 to 130 5 mL 3.5 mL/h 9 mL/h 1.8 mL/h 4.5 mL/h 60 to 65 131 to 143 5.6 mL 3.8 mL/h 10 mL/h 1.9 mL/h 5 mL/h 66 to 71 144 to 157 6.2 mL 4 mL/h 11 mL/h 2 mL/h 5.5 mL/h 72 to 78 158 to 172 6.8 mL 4.5 mL/h 12 mL/h 2.3 mL/h 6 mL/h 79 to 84 173 to 185 7.3 mL 5 mL/h 13 mL/h 2.5 mL/h 6.5 mL/h 85 to 90 186 to 198 7.9 mL 5.3 mL/h 14 mL/h 2.7 mL/h 7 mL/h 91 to 96 199 to 212 8.5 mL 5.6 mL/h 15 mL/h 2.8 mL/h 7.5 mL/h 97 to 103 213 to 227 9 mL 6 mL/h 16 mL/h 3 mL/h 8 mL/h 104 to 109 228 to 240 9.5 mL 6.4 mL/h 17 mL/h 3.2 mL/h 8.5 mL/h 110 to 115 241 to 253 10.2 mL 6.8 mL/h 18 mL/h 3.4 mL/h 9 mL/h 116 to 121 254 to 267 10.7 mL 7 mL/h 19 mL/h 3.5 mL/h 9.5 mL/h >121 >267 11.3 mL 7.5 mL/h 20 mL/h 3.7 mL/h 10 mL/h - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Treatment with eptifibatide is contraindicated in patients with:
- A history of bleeding diathesis, or evidence of active abnormal bleeding within the previous 30 days
- Severe hypertension (systolic blood pressure >200 mm Hg or diastolic blood pressure >110 mm Hg) not adequately controlled on antihypertensive therapy
- Major surgery within the preceding 6 weeks
- History of stroke within 30 days or any history of hemorrhagic stroke
- Current or planned administration of another parenteral GP IIb/IIIa inhibitor
- Dependency on renal dialysis
- Hypersensitivity to eptifibatide or any component of the product (hypersensitivity reactions that occurred included anaphylaxis and urticaria).
-
5 WARNINGS AND PRECAUTIONS
5.1 Bleeding
Bleeding is the most common complication encountered during eptifibatide therapy. Administration of eptifibatide is associated with an increase in major and minor bleeding, as classified by the criteria of the Thrombolysis in Myocardial Infarction Study group (TIMI) [see Adverse Reactions (6.1)]. Most major bleeding associated with eptifibatide has been at the arterial access site for cardiac catheterization or from the gastrointestinal or genitourinary tract. Minimize the use of arterial and venous punctures, intramuscular injections and the use of urinary catheters, nasotracheal intubation and nasogastric tubes. When obtaining intravenous access, avoid non-compressible sites (e.g., subclavian or jugular veins).
Use of Thrombolytics, Anticoagulants and Other Antiplatelet Agents
Risk factors for bleeding include older age, a history of bleeding disorders and concomitant use of drugs that increase the risk of bleeding (thrombolytics, oral anticoagulants, nonsteroidal anti-inflammatory drugs and P2Y12 inhibitors). Concomitant treatment with other inhibitors of platelet receptor glycoprotein (GP) IIb/IIIa should be avoided. In patients treated with heparin, bleeding can be minimized by close monitoring of the aPTT and ACT [see Dosage and Administration (2)].
Care of the Femoral Artery Access Site in Patients Undergoing Percutaneous Coronary Intervention (PCI)
In patients undergoing PCI, treatment with eptifibatide is associated with an increase in major and minor bleeding at the site of arterial sheath placement. After PCI, eptifibatide infusion should be continued until hospital discharge or up to 18 to 24 hours, whichever comes first. Heparin use is discouraged after the PCI procedure. Early sheath removal is encouraged while eptifibatide is being infused. Prior to removing the sheath, it is recommended that heparin be discontinued for 3 to 4 hours and an aPTT of <45 seconds or ACT <150 seconds be achieved. In any case, both heparin and eptifibatide should be discontinued and sheath hemostasis should be achieved at least 2 to 4 hours before hospital discharge. If bleeding at access site cannot be controlled with pressure, infusion of eptifibatide and heparin should be discontinued immediately.
5.2 Thrombocytopenia
There have been reports of acute, profound thrombocytopenia (immune-mediated and non-immune mediated) with eptifibatide. In the event of acute profound thrombocytopenia or a confirmed platelet decrease to <100,000/mm3, discontinue eptifibatide and heparin (unfractionated or low-molecular weight). Monitor serial platelet counts, assess the presence of drug-dependent antibodies and treat as appropriate [see Adverse Reactions (6.1)].
There has been no clinical experience with eptifibatide initiated in patients with a baseline platelet count <100,000/mm3. If a patient with low platelet counts is receiving eptifibatide, their platelet count should be monitored closely.
-
6 ADVERSE REACTIONS
The following serious adverse reaction is also discussed elsewhere in the labeling:
- Bleeding [see Contraindications (4) and Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 16,782 patients were treated in the Phase III clinical trials (PURSUIT, ESPRIT and IMPACT II) [see Clinical Trials (14)]. These 16,782 patients had a mean age of 62 years (range: 20 to 94 years). Eighty-nine percent of the patients were Caucasian, with the remainder being predominantly Black (5%) and Hispanic (5%). Sixty-eight percent were men. Because of the different regimens used in PURSUIT, IMPACT II and ESPRIT, data from the 3 studies were not pooled.
Bleeding and hypotension were the most commonly reported adverse reactions (incidence ≥5% and greater than placebo) in the eptifibatide controlled clinical trial database.
Bleeding
The incidence of bleeding and transfusions in the PURSUIT and ESPRIT studies are shown in Table 2. Bleeding was classified as major or minor by the criteria of the TIMI study group. Major bleeding consisted of intracranial hemorrhage and other bleeding that led to decreases in hemoglobin greater than 5 g/dL. Minor bleeding included spontaneous gross hematuria, spontaneous hematemesis, other observed blood loss with a hemoglobin decrease of more than 3 g/dL and other hemoglobin decreases that were greater than 4 g/dL but less than 5 g/dL. In patients who received transfusions, the corresponding loss in hemoglobin was estimated through an adaptation of the method of Landefeld et al.
Table 2: Bleeding and Transfusions in the PURSUIT and ESPRIT Studies PURSUIT (ACS)
Placebo
n (%)Eptifibatide
180/2
n (%)Patients
4696
4679
Major bleeding*
425 (9.3%)
498 (10.8%)
Minor bleeding*
347 (7.6%)
604 (13.1%)
Requiring transfusions†
490 (10.4%)
601 (12.8%)
ESPRIT (PCI)
Placebo
n (%)Eptifibatide
180/2/180
n (%)Patients
1024
1040
Major bleeding*
4 (0.4%)
13 (1.3%)
Minor bleeding*
18 (2%)
29 (3%)
Requiring transfusions†
11 (1.1%)
16 (1.5%)
Note: Denominator is based on patients for whom data are available. * For major and minor bleeding, patients are counted only once according to the most severe classification. † Includes transfusions of whole blood, packed red blood cells, fresh frozen plasma, cryoprecipitate, platelets and autotransfusion during the initial hospitalization. The majority of major bleeding reactions in the ESPRIT study occurred at the vascular access site (1 and 8 patients, or 0.1% and 0.8% in the placebo and eptifibatide groups, respectively). Bleeding at "other" locations occurred in 0.2% and 0.4% of patients, respectively.
In the PURSUIT study, the greatest increase in major bleeding in eptifibatide-treated patients compared to placebo-treated patients was also associated with bleeding at the femoral artery access site (2.8% versus 1.3%). Oropharyngeal (primarily gingival), genitourinary, gastrointestinal and retroperitoneal bleeding were also seen more commonly in eptifibatide-treated patients compared to placebo-treated patients.
Among patients experiencing a major bleed in the IMPACT II study, an increase in bleeding on eptifibatide versus placebo was observed only for the femoral artery access site (3.2% versus 2.8%).
Table 3 displays the incidence of TIMI major bleeding according to the cardiac procedures carried out in the PURSUIT study. The most common bleeding complications were related to cardiac revascularization (CABG-related or femoral artery access site bleeding). A corresponding table for ESPRIT is not presented, as every patient underwent PCI in the ESPRIT study and only 11 patients underwent CABG.
Table 3: Major Bleeding by Procedures in the PURSUIT Study Placebo
n (%)Eptifibatide
180/2
n (%)Patients
4577
4604
Overall incidence of major bleeding
425 (9.3%)
498 (10.8%)
Breakdown by procedure:
CABG
375 (8.2%)
377 (8.2%)
Angioplasty without CABG
27 (0.6%)
64 (1.4%)
Angiography without angioplasty or CABG
11 (0.2%)
29 (0.6%)
Medical therapy only
12 (0.3%)
28 (0.6%)
Note: Denominators are based on the total number of patients whose TIMI classification was resolved. In the PURSUIT and ESPRIT studies, the risk of major bleeding with eptifibatide increased as patient weight decreased. This relationship was most apparent for patients weighing less than 70 kg.
Bleeding resulting in discontinuation of the study drug was more frequent among patients receiving eptifibatide than placebo (4.6% versus 0.9% in ESPRIT, 8% versus 1% in PURSUIT, 3.5% versus 1.9% in IMPACT II).
Intracranial Hemorrhage and Stroke
Intracranial hemorrhage was rare in the PURSUIT, IMPACT II and ESPRIT clinical studies. In the PURSUIT study, 3 patients in the placebo group, 1 patient in the group treated with eptifibatide 180/1.3 and 5 patients in the group treated with eptifibatide 180/2 experienced a hemorrhagic stroke. The overall incidence of stroke was 0.5% in patients receiving eptifibatide 180/1.3, 0.7% in patients receiving eptifibatide 180/2 and 0.8% in placebo patients.
In the IMPACT II study, intracranial hemorrhage was experienced by 1 patient treated with eptifibatide 135/0.5, 2 patients treated with eptifibatide 135/0.75 and 2 patients in the placebo group. The overall incidence of stroke was 0.5% in patients receiving 135/0.5 eptifibatide, 0.7% in patients receiving eptifibatide 135/0.75 and 0.7% in the placebo group.
In the ESPRIT study, there were 3 hemorrhagic strokes, 1 in the placebo group and 2 in the eptifibatide group. In addition there was 1 case of cerebral infarction in the eptifibatide group.
Immunogenicity/Thrombocytopenia
The potential for development of antibodies to eptifibatide has been studied in 433 subjects. Eptifibatide was nonantigenic in 412 patients receiving a single administration of eptifibatide (135-mcg/kg bolus followed by a continuous infusion of either 0.5 mcg/kg/min or 0.75 mcg/kg/min), and in 21 subjects to whom eptifibatide (135-mcg/kg bolus followed by a continuous infusion of 0.75 mcg/kg/min) was administered twice, 28 days apart. In both cases, plasma for antibody detection was collected approximately 30 days after each dose. The development of antibodies to eptifibatide at higher doses has not been evaluated.
In patients with suspected eptifibatide -related immune-mediated thrombocytopenia, IgG antibodies that react with the GP IIb/IIIa complex were identified in the presence of eptifibatide and in eptifibatide-naïve patients. These findings suggest acute thrombocytopenia after the administration of eptifibatide can develop as a result of naturally occurring drug-dependent antibodies or those induced by prior exposure to eptifibatide. Similar antibodies were identified with other GP IIb/IIIa ligand-mimetic agents. Immune-mediated thrombocytopenia with eptifibatide may be associated with hypotension and/or other signs of hypersensitivity.
In the PURSUIT and IMPACT II studies, the incidence of thrombocytopenia (<100,000/mm3 or ≥50% reduction from baseline) and the incidence of platelet transfusions were similar between patients treated with eptifibatide and placebo. In the ESPRIT study, the incidence was 0.6% in the placebo group and 1.2% in the eptifibatide group.
Other Adverse Reactions
In the PURSUIT and ESPRIT studies, the incidence of serious nonbleeding adverse reactions was similar in patients receiving placebo or eptifibatide (19% and 19%, respectively, in PURSUIT; 6% and 7%, respectively, in ESPRIT). In PURSUIT, the only serious nonbleeding adverse reaction that occurred at a rate of at least 1% and was more common with eptifibatide than placebo (7% versus 6%) was hypotension. Most of the serious nonbleeding adverse reactions consisted of cardiovascular reactions typical of a UA population. In the IMPACT II study, serious nonbleeding adverse reactions that occurred in greater than 1% of patients were uncommon and similar in incidence between placebo- and eptifibatide-treated patients.
Discontinuation of study drug due to adverse reactions other than bleeding was uncommon in the PURSUIT, IMPACT II and ESPRIT studies, with no single reaction occurring in >0.5% of the study population (except for 'other' in the ESPRIT study).
6.2 Postmarketing Experience
Because the reactions below are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported in postmarketing experience, primarily with eptifibatide in combination with heparin and aspirin: cerebral, GI and pulmonary hemorrhage. Fatal bleeding reactions have been reported. Acute profound thrombocytopenia, as well as immune-mediated thrombocytopenia, has been reported [see Adverse Reactions (6.1)].
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Teratology studies have been performed by continuous intravenous infusion of eptifibatide in pregnant rats at total daily doses of up to 72 mg/kg/day (about 4 times the recommended maximum daily human dose on a body surface area basis) and in pregnant rabbits at total daily doses of up to 36 mg/kg/day (also about 4 times the recommended maximum daily human dose on a body surface area basis). These studies revealed no evidence of harm to the fetus due to eptifibatide. There are, however, no adequate and well-controlled studies in pregnant women with eptifibatide. Because animal reproduction studies are not always predictive of human response, eptifibatide should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether eptifibatide is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when eptifibatide is administered to a nursing mother.
8.4 Pediatric Use
Safety and effectiveness of eptifibatide in pediatric patients have not been studied.
8.5 Geriatric Use
The PURSUIT and IMPACT II clinical studies enrolled patients up to the age of 94 years (45% were age 65 and over; 12% were age 75 and older). There was no apparent difference in efficacy between older and younger patients treated with eptifibatide. The incidence of bleeding complications was higher in the elderly in both placebo and eptifibatide groups and the incremental risk of eptifibatide-associated bleeding was greater in the older patients. No dose adjustment was made for elderly patients, but patients over 75 years of age had to weigh at least 50 kg to be enrolled in the PURSUIT study; no such limitation was stipulated in the ESPRIT study [see Adverse Reactions (6.1)].
8.6 Renal Impairment
Approximately 50% of eptifibatide is cleared by the kidney in patients with normal renal function. Total drug clearance is decreased by approximately 50% and steady-state plasma eptifibatide concentrations are doubled in patients with an estimated CrCl <50 mL/min (using the Cockcroft-Gault equation). Therefore, the infusion dose should be reduced to 1 mcg/kg/min in such patients [see Dosage and Administration (2)]. The safety and efficacy of eptifibatide in patients dependent on dialysis has not been established.
-
10 OVERDOSAGE
There has been only limited experience with overdosage of eptifibatide. There were 8 patients in the IMPACT II study, 9 patients in the PURSUIT study and no patients in the ESPRIT study who received bolus doses and/or infusion doses more than double those called for in the protocols. None of these patients experienced an intracranial bleed or other major bleeding.
Eptifibatide was not lethal to rats, rabbits, or monkeys when administered by continuous intravenous infusion for 90 minutes at a total dose of 45 mg/kg (about 2 to 5 times the recommended maximum daily human dose on a body surface area basis). Symptoms of acute toxicity were loss of righting reflex, dyspnea, ptosis and decreased muscle tone in rabbits and petechial hemorrhages in the femoral and abdominal areas of monkeys.
From in vitro studies, eptifibatide is not extensively bound to plasma proteins and thus may be cleared from plasma by dialysis.
-
11 DESCRIPTION
Eptifibatide is a cyclic heptapeptide containing 6 amino acids and 1 mercaptopropionyl (des-amino cysteinyl) residue. An interchain disulfide bridge is formed between the cysteine amide and the mercaptopropionyl moieties. Chemically it is N6-(aminoiminomethyl)-N2-(3-mercapto-1-oxopropyl)-L-lysylglycyl-L-α-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide, cyclic (1→6)-disulfide. Eptifibatide binds to the platelet receptor glycoprotein (GP) IIb/IIIa of human platelets and inhibits platelet aggregation.
The eptifibatide peptide is produced by solution-phase peptide synthesis, and is purified by preparative reverse-phase liquid chromatography and lyophilized. The structural formula is:
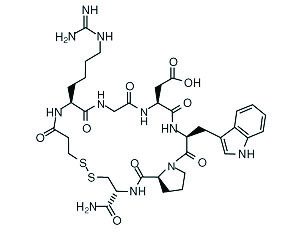
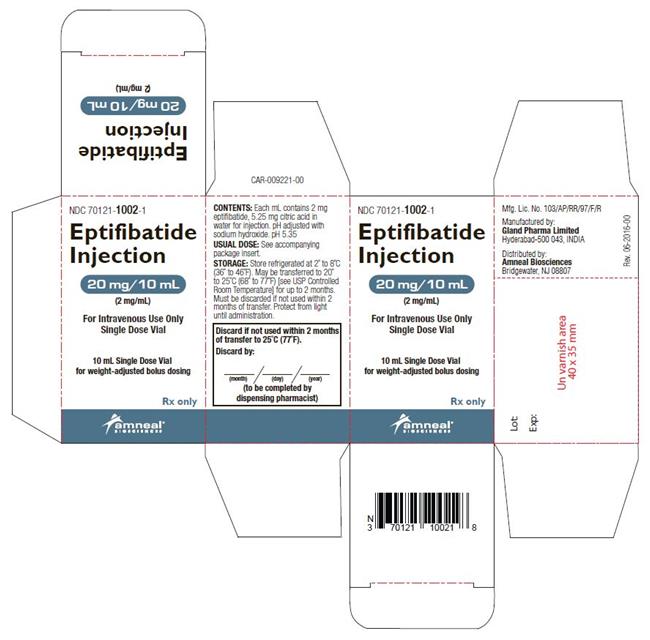
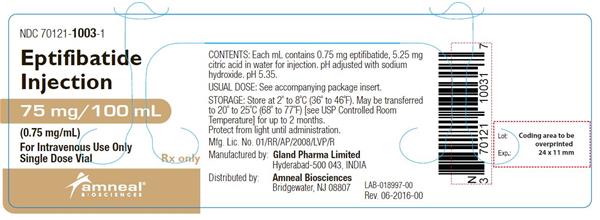
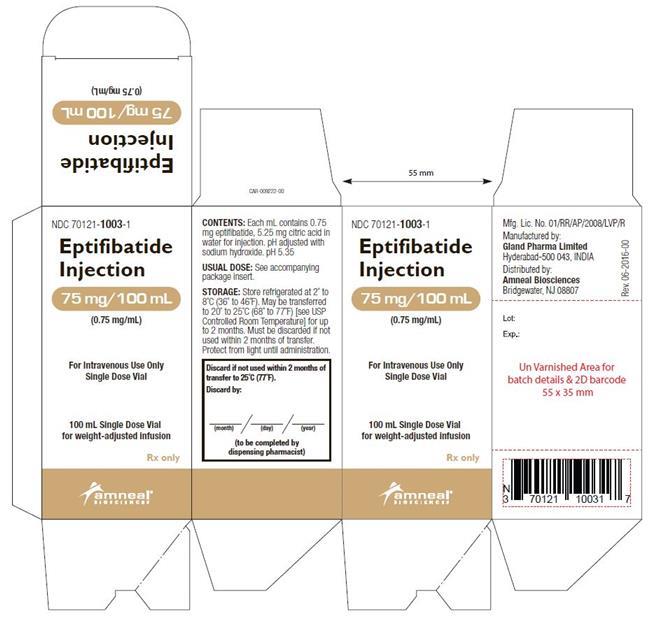
Eptifibatide injection is a clear, colorless, sterile, non-pyrogenic solution for intravenous (IV) use with an empirical formula of C35H49N11O9S2 and a molecular weight of 831.96. Each 10-mL vial contains 2 mg/mL of eptifibatide and each 100-mL vial contains 0.75 mg/mL of eptifibatide. Each vial of either size also contains 5.25 mg/mL citric acid in water for injection and sodium hydroxide to adjust the pH to 5.35.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Eptifibatide reversibly inhibits platelet aggregation by preventing the binding of fibrinogen, von Willebrand factor and other adhesive ligands to GP IIb/IIIa. When administered intravenously, eptifibatide inhibits ex vivo platelet aggregation in a dose- and concentration-dependent manner. Platelet aggregation inhibition is reversible following cessation of the eptifibatide infusion; this is thought to result from dissociation of eptifibatide from the platelet.
12.2 Pharmacodynamics
Infusion of eptifibatide into baboons caused a dose-dependent inhibition of ex vivo platelet aggregation, with complete inhibition of aggregation achieved at infusion rates greater than 5 mcg/kg/min. In a baboon model that is refractory to aspirin and heparin, doses of eptifibatide that inhibit aggregation prevented acute thrombosis with only a modest prolongation (2- to 3-fold) of the bleeding time. Platelet aggregation in dogs was also inhibited by infusions of eptifibatide, with complete inhibition at 2 mcg/kg/min. This infusion dose completely inhibited canine coronary thrombosis induced by coronary artery injury (Folts model).
Human pharmacodynamic data were obtained in healthy subjects and in patients presenting with UA or NSTEMI and/or undergoing percutaneous coronary intervention. Studies in healthy subjects enrolled only males; patient studies enrolled approximately one-third women. In these studies, eptifibatide inhibited ex vivo platelet aggregation induced by adenosine diphosphate (ADP) and other agonists in a dose- and concentration-dependent manner. The effect of eptifibatide was observed immediately after administration of a 180-mcg/kg intravenous bolus. Table 4 shows the effects of dosing regimens of eptifibatide used in the IMPACT II and PURSUIT studies on ex vivo platelet aggregation induced by 20 µM ADP in PPACK-anticoagulated platelet-rich plasma and on bleeding time. The effects of the dosing regimen used in ESPRIT on platelet aggregation have not been studied.
Table 4: Platelet Inhibition and Bleeding Time PURSUIT
180/2*Inhibition of platelet aggregation 15 min after bolus
84%
Inhibition of platelet aggregation at steady-state
>90%
Bleeding-time prolongation at steady-state
<5x
Inhibition of platelet aggregation 4h after infusion discontinuation
<50%
Bleeding-time prolongation 6h after infusion discontinuation
1.4x
* 180-mcg/kg bolus followed by a continuous infusion of 2 mcg/kg/min. The eptifibatide dosing regimen used in the ESPRIT study included two 180-mcg/kg bolus doses given 10 minutes apart combined with a continuous 2-mcg/kg/min infusion.
When administered alone, eptifibatide has no measurable effect on PT or aPTT.
There were no important differences between men and women or between age groups in the pharmacodynamic properties of eptifibatide. Differences among ethnic groups have not been assessed.
12.3 Pharmacokinetics
The pharmacokinetics of eptifibatide are linear and dose-proportional for bolus doses ranging from 90 to 250 mcg/kg and infusion rates from 0.5 to 3 mcg/kg/min. Plasma elimination half-life is approximately 2.5 hours. Administration of a single 180-mcg/kg bolus combined with an infusion produces an early peak level, followed by a small decline prior to attaining steady-state (within 4 to 6 hours). This decline can be prevented by administering a second 180-mcg/kg bolus 10 minutes after the first. The extent of eptifibatide binding to human plasma protein is about 25%. Clearance in patients with coronary artery disease is about 55 mL/kg/h. In healthy subjects, renal clearance accounts for approximately 50% of total body clearance, with the majority of the drug excreted in the urine as eptifibatide, deaminated eptifibatide and other, more polar metabolites. No major metabolites have been detected in human plasma.
Special Populations
Geriatric
Patients in clinical studies were older (range: 20 to 94 years) than those in the clinical pharmacology studies. Elderly patients with coronary artery disease demonstrated higher plasma levels and lower total body clearance of eptifibatide when given the same dose as younger patients. Limited data are available on lighter weight (<50 kg) patients over 75 years of age.
Renal Impairment
In patients with moderate to severe renal insufficiency (CrCl <50 mL/min using the Cockcroft-Gault equation), the clearance of eptifibatide is reduced by approximately 50% and steady-state plasma levels approximately doubled [see Use in Specific Populations (8.6) and Dosage and Administration (2)].
Hepatic Impairment
No studies have been conducted in patients with hepatic impairment.
Gender
Males and females have not demonstrated any clinically significant differences in the pharmacokinetics of eptifibatide.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of eptifibatide. Eptifibatide was not genotoxic in the Ames test, the mouse lymphoma cell (L 5178Y, TK+/-) forward mutation test, the human lymphocyte chromosome aberration test, or the mouse micronucleus test. Administered by continuous intravenous infusion at total daily doses up to 72 mg/kg/day (about 4 times the recommended maximum daily human dose on a body surface area basis), eptifibatide had no effect on fertility and reproductive performance of male and female rats.
-
14 CLINICAL STUDIES
Eptifibatide was studied in 3 placebo-controlled, randomized studies. PURSUIT evaluated patients with acute coronary syndromes: UA or NSTEMI. Two other studies, ESPRIT and IMPACT II, evaluated patients about to undergo a PCI. Patients underwent primarily balloon angioplasty in IMPACT II and intracoronary stent placement, with or without angioplasty, in ESPRIT.
14.1 Non-ST-Segment Elevation Acute Coronary Syndrome
Non-ST-segment elevation acute coronary syndrome is defined as prolonged (≥10 minutes) symptoms of cardiac ischemia within the previous 24 hours associated with either ST-segment changes (elevations between 0.6 mm and 1 mm or depression >0.5 mm), T-wave inversion (>1 mm), or positive CK-MB. This definition includes "unstable angina" and "NSTEMI" but excludes MI that is associated with Q waves or greater degrees of ST-segment elevation.
PURSUIT (Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Eptifibatide Therapy)
PURSUIT was a 726-center, 27-country, double-blind, randomized, placebo-controlled study in 10,948 patients presenting with UA or NSTEMI. Patients could be enrolled only if they had experienced cardiac ischemia at rest (≥10 minutes) within the previous 24 hours and had either ST-segment changes (elevations between 0.6 mm and 1 mm or depression >0.5 mm), T-wave inversion (>1 mm), or increased CK-MB. Important exclusion criteria included a history of bleeding diathesis, evidence of abnormal bleeding within the previous 30 days, uncontrolled hypertension, major surgery within the previous 6 weeks, stroke within the previous 30 days, any history of hemorrhagic stroke, serum creatinine >2 mg/dL, dependency on renal dialysis, or platelet count <100,000/mm3.
Patients were randomized to placebo, to eptifibatide 180-mcg/kg bolus followed by a 2-mcg/kg/min infusion (180/2), or to eptifibatide 180-mcg/kg bolus followed by a 1.3-mcg/kg/min infusion (180/1.3). The infusion was continued for 72 hours, until hospital discharge, or until the time of CABG, whichever occurred first, except that if PCI was performed, the eptifibatide infusion was continued for 24 hours after the procedure, allowing for a duration of infusion up to 96 hours.
The lower-infusion-rate arm was stopped after the first interim analysis when the 2 active-treatment arms appeared to have the same incidence of bleeding.
Patient age ranged from 20 to 94 (mean 63) years, and 65% were male. The patients were 89% Caucasian, 6% Hispanic and 5% Black, recruited in the United States and Canada (40%), Western Europe (39%), Eastern Europe (16%) and Latin America (5%).
This was a "real world" study; each patient was managed according to the usual standards of the investigational site; frequencies of angiography, PCI and CABG therefore differed widely from site to site and from country to country. Of the patients in PURSUIT, 13% were managed with PCI during drug infusion, of whom 50% received intracoronary stents; 87% were managed medically (without PCI during drug infusion).
The majority of patients received aspirin (75 to 325 mg once daily). Heparin was administered intravenously or subcutaneously, at the physician"s discretion, most commonly as an intravenous bolus of 5000 units followed by a continuous infusion of 1000 units/h. For patients weighing less than 70 kg, the recommended heparin bolus dose was 60 units/kg followed by a continuous infusion of 12 units/kg/h. A target aPTT of 50 to 70 seconds was recommended. A total of 1250 patients underwent PCI within 72 hours after randomization, in which case they received intravenous heparin to maintain an ACT of 300 to 350 seconds.
The primary endpoint of the study was the occurrence of death from any cause or new MI (evaluated by a blinded Clinical Endpoints Committee) within 30 days of randomization.
Compared to placebo, eptifibatide administered as a 180-mcg/kg bolus followed by a 2mcg/kg/min infusion significantly (p=0.042) reduced the incidence of endpoint events (see Table 6). The reduction in the incidence of endpoint events in patients receiving eptifibatide was evident early during treatment, and this reduction was maintained through at least 30 days (see Figure 1). Table 5 also shows the incidence of the components of the primary endpoint, death (whether or not preceded by an MI) and new MI in surviving patients at 30 days.
Table 5: Clinical Events in the PURSUIT Study Death or MI
Placebo
(n=4739)
n (%)Eptifibatide
(180 mcg/kg bolus then
2 mcg/kg/min infusion)
(n=4722)
n (%)p-value
3 days
359 (7.6%)
279 (5.9%)
0.001
7 days
552 (11.6%)
477 (10.1%)
0.016
30 days
Death or MI (primary endpoint)
745 (15.7%)
672 (14.2%)
0.042
Death
177 (3.7%)
165 (3.5%)
Nonfatal MI
568 (12%)
507 (10.7%)
Figure 1: Kaplan-Meier Plot of Time to Death or Myocardial Infarction Within 30 Days of Randomization in the PURSUIT Study
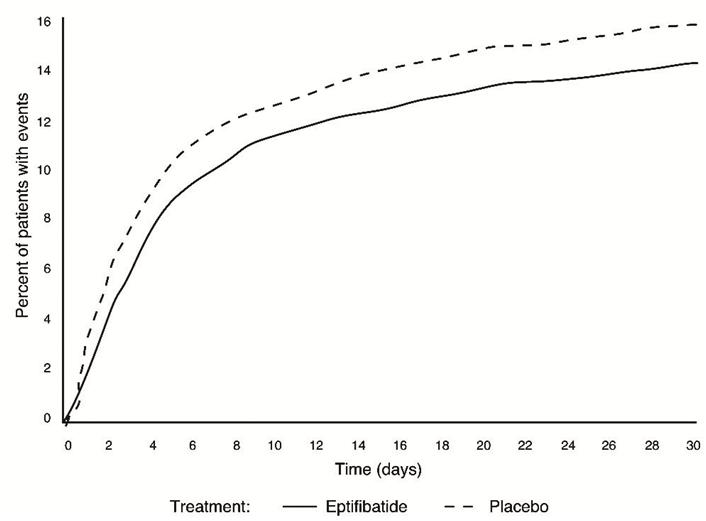
Treatment with eptifibatide prior to determination of patient management strategy reduced clinical events regardless of whether patients ultimately underwent diagnostic catheterization, revascularization (i.e., PCI or CABG surgery) or continued to receive medical management alone. Table 6 shows the incidence of death or MI within 72 hours.
Table 6: Clinical Events (Death or MI) in the PURSUIT Study Within 72 Hours of Randomization Placebo
Eptifibatide
(180 mcg/kg bolus
then 2 mcg/kg/min
infusion)Overall patient population
n=4739
n=4722
– At 72 hours
7.6%
5.9%
Patients undergoing early PCI
n=631
n=619
– Pre-procedure (nonfatal MI only)
5.5%
1.8%
– At 72 hours
14.4%
9%
Patients not undergoing early PCI
n=4108
n=4103
– At 72 hours
6.5%
5.4%
All of the effect of eptifibatide was established within 72 hours (during the period of drug infusion), regardless of management strategy. Moreover, for patients undergoing early PCI, a reduction in events was evident prior to the procedure.
An analysis of the results by sex suggests that women who would not routinely be expected to undergo PCI receive less benefit from eptifibatide (95% confidence limits for relative risk of 0.94 to 1.28) than do men (0.72 to 0.9). This difference may be a true treatment difference, the effect of other differences in these subgroups, or a statistical anomaly. No differential outcomes were seen between male and female patients undergoing PCI (see results for ESPRIT).
Follow-up data were available through 165 days for 10,611 patients enrolled in the PURSUIT trial (96.9% of the initial enrollment). This follow-up included 4566 patients who received eptifibatide at the 180/2 dose. As reported by the investigators, the occurrence of death from any cause or new MI for patients followed for at least 165 days was reduced from 13.6% with placebo to 12.1% with eptifibatide 180/2.
14.2 Percutaneous Coronary Intervention (PCI)
IMPACT II (Eptifibatide to Minimize Platelet Aggregation and Prevent Coronary Thrombosis II)
IMPACT II was a multicenter, double-blind, randomized, placebo-controlled study conducted in the United States in 4010 patients undergoing PCI. Major exclusion criteria included a history of bleeding diathesis, major surgery within 6 weeks of treatment, gastrointestinal bleeding within 30 days, any stroke or structural CNS abnormality, uncontrolled hypertension, PT >1.2 times control, hematocrit <30%, platelet count <100,000/mm3 and pregnancy.
Patient age ranged from 24 to 89 (mean 60) years, and 75% were male. The patients were 92% Caucasian, 5% Black and 3% Hispanic. Forty-one percent of the patients underwent PCI for ongoing ACS. Patients were randomly assigned to 1 of 3 treatment regimens, each incorporating a bolus dose initiated immediately prior to PCI followed by a continuous infusion lasting 20 to 24 hours:
1) 135-mcg/kg bolus followed by a continuous infusion of 0.5 mcg/kg/min of eptifibatide (135/0.5);
2) 135-mcg/kg bolus followed by a continuous infusion of 0.75 mcg/kg/min of eptifibatide (135/0.75); or
3) a matching placebo bolus followed by a matching placebo continuous infusion.Each patient received aspirin and an intravenous heparin bolus of 100 units/kg, with additional bolus infusions of up to 2000 additional units of heparin every 15 minutes to maintain an ACT of 300 to 350 seconds.
The primary endpoint was the composite of death, MI, or urgent revascularization, analyzed at 30 days after randomization in all patients who received at least 1 dose of study drug.
As shown in Table 7, each eptifibatide regimen reduced the rate of death, MI, or urgent intervention, although at 30 days, this finding was statistically significant only in the lower-dose eptifibatide group. As in the PURSUIT study, the effects of eptifibatide were seen early and persisted throughout the 30-day period.
Table 7: Clinical Events in the IMPACT II Study Placebo
n (%)Eptifibatide
(135 mcg/kg
bolus then
0.5 mcg/kg/min
infusion)
n (%)Eptifibatide
(135 mcg/kg
bolus then
0.75 mcg/kg/min
infusion)
n (%)Patients
1285
1300
1286
Abrupt Closure
65 (5.1%)
36 (2.8%)
43 (3.3%)
p-value versus placebo
0.003
0.03
Death, MI, or Urgent Intervention
24 hours
123 (9.6%)
86 (6.6%)
89 (6.9%)
p-value versus placebo
0.006
0.014
48 hours
131 (10.2%)
99 (7.6%)
102 (7.9%)
p-value versus placebo
0.021
0.045
30 days (primary endpoint)
149 (11.6%)
118 (9.1%)
128 (10%)
p-value versus placebo
0.035
0.179
Death or MI
30 days
110 (8.6%)
89 (6.8%)
95 (7.4%)
p-value versus placebo
0.102
0.272
6 months
151 (11.9%)*
136 (10.6%)*
130 (10.3%)*
p-value versus placebo
0.297
0.182
* Kaplan-Meier estimate of event rate. ESPRIT (Enhanced Suppression of the Platelet IIb/IIIa Receptor with Eptifibatide Therapy)
The ESPRIT study was a multicenter, double-blind, randomized, placebo-controlled study conducted in the United States and Canada that enrolled 2064 patients undergoing elective or urgent PCI with intended intracoronary stent placement. Exclusion criteria included MI within the previous 24 hours, ongoing chest pain, administration of any oral antiplatelet or oral anticoagulant other than aspirin within 30 days of PCI (although loading doses of thienopyridine on the day of PCI were encouraged), planned PCI of a saphenous vein graft or subsequent “staged” PCI, prior stent placement in the target lesion, PCI within the previous 90 days, a history of bleeding diathesis, major surgery within 6 weeks of treatment, gastrointestinal bleeding within 30 days, any stroke or structural CNS abnormality, uncontrolled hypertension, PT >1.2 times control, hematocrit <30%, platelet count <100,000/mm3 and pregnancy.
Patient age ranged from 24 to 93 (mean 62) years, and 73% of patients were male. The study enrolled 90% Caucasian, 5% African American, 2% Hispanic and 1% Asian patients. Patients received a wide variety of stents. Patients were randomized either to placebo or eptifibatide administered as an intravenous bolus of 180 mcg/kg followed immediately by a continuous infusion of 2 mcg/kg/min, and a second bolus of 180 mcg/kg administered 10 minutes later (180/2/180). Eptifibatide infusion was continued for 18 to 24 hours after PCI or until hospital discharge, whichever came first. Each patient received at least 1 dose of aspirin (162 to 325 mg) and 60 units/kg of heparin as a bolus (not to exceed 6000 units) if not already receiving a heparin infusion. Additional boluses of heparin (10 to 40 units/kg) could be administered in order to reach a target ACT between 200 and 300 seconds.
The primary endpoint of the ESPRIT study was the composite of death, MI, urgent target vessel revascularization (UTVR) and “bailout” to open-label eptifibatide due to a thrombotic complication of PCI (TBO) (e.g., visible thrombus, “no reflow,” or abrupt closure) at 48 hours. MI, UTVR and TBO were evaluated by a blinded Clinical Events Committee.
As shown in Table 8, the incidence of the primary endpoint and selected secondary endpoints was significantly reduced in patients who received eptifibatide. A treatment benefit in patients who received eptifibatide was seen by 48 hours and at the end of the 30-day observation period.
Table 8: Clinical Events in the ESPRIT Study Placebo
(n=1024)Eptifibatide*
(n=1040)Relative Risk
(95% CI)p-value
Death, MI, UTVR, or Thrombotic “Bailout”
48 hours (primary endpoint)
108 (10.5%)
69 (6.6%)
0.629 (0.471, 0.84)
0.0015
30 days
120 (11.7%)
78 (7.5%)
0.64 (0.488, 0.84)
0.0011
Death, MI, UTVR
48 hours
95 (9.3%)
62 (6%)
0.643 (0.472, 0.875)
0.0045
30 days (key secondary endpoint)
107 (10.4%)
71 (6.8%)
0.653 (0.49, 0.871)
0.0034
Death or MI
48 hours
94 (9.2%)
57 (5.5%)
0.597 (0.435, 0.82)
0.0013
30 days
104 (10.2%)
66 (6.3%)
0.625 (0.465, 0.84)
0.0016
* Eptifibatide was administered as 180 mcg/kg boluses at times 0 and 10 minutes and an infusion at 2 mcg/kg/min. The need for thrombotic “bailout” was significantly reduced with eptifibatide at 48 hours (2.1% for placebo, 1% for eptifibatide; p=0.029). Consistent with previous studies of GP IIb/IIIa inhibitors, most of the benefit achieved acutely with eptifibatide was in the reduction of MI. Eptifibatide reduced the occurrence of MI at 48 hours from 9% for placebo to 5.4% (p=0.0015) and maintained that effect with significance at 30 days.
There was no treatment difference with respect to sex in ESPRIT. Eptifibatide reduced the incidence of the primary endpoint in both men (95% confidence limits for relative risk: 0.54, 1.07) and women (0.24, 0.72) at 48 hours.
Follow-up (12-month) mortality data were available for 2024 patients (1017 on eptifibatide) enrolled in the ESPRIT trial (98.1% of the initial enrollment). Twelve-month clinical event data were available for 1964 patients (988 on eptifibatide), representing 95.2% of the initial enrollment. As shown in Table 9, the treatment effect of eptifibatide seen at 48 hours and 30 days appeared preserved at 6 months and 1 year. Most of the benefit was in reduction of MI.
Table 9: Clinical Events at 6 Months and 1 Year in the ESPRIT Study Placebo
(n=1024)Eptifibatide
(n=1040)Hazard Ratio
(95% CI)Death, MI, or Target Vessel Revascularization
6 months
187 (18.5%)
146 (14.3%)
0.744 (0.599, 0.924)
1 year
222 (22.1%)
178 (17.5%)
0.762 (0.626, 0.929)
Death, MI
6 months
117 (11.5%)
77 (7.4%)
0.631 (0.473, 0.841)
1 year
126 (12.4%)
83 (8%)
0.63 (0.478, 0.832)
Percentages are Kaplan-Meier event rates. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Eptifibatide injection is supplied as a sterile solution in 10-mL vials containing 20 mg of eptifibatide (NDC: 70121-1002-1) and 100-mL vials containing 75 mg of eptifibatide (NDC: 70121-1003-1).
16.2 Storage
Vials should be stored refrigerated at 2° to 8°C (36° to 46°F). Vials may be transferred to room temperature storage * for a period not to exceed 2 months. Upon transfer, vial cartons must be marked by the dispensing pharmacist with a "DISCARD BY" date (2 months from the transfer date or the labeled expiration date, whichever comes first).
* Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EPTIFIBATIDE
eptifibatide injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70121-1002 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPTIFIBATIDE (UNII: NA8320J834) (EPTIFIBATIDE - UNII:NA8320J834) EPTIFIBATIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 5.25 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (clear, colorless) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70121-1002-1 1 in 1 CARTON 12/08/2016 1 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205581 12/08/2016 EPTIFIBATIDE
eptifibatide injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70121-1003 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPTIFIBATIDE (UNII: NA8320J834) (EPTIFIBATIDE - UNII:NA8320J834) EPTIFIBATIDE 0.75 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 5.25 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (clear, colorless) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70121-1003-1 1 in 1 CARTON 12/08/2016 1 100 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205581 12/08/2016 Labeler - Amneal Pharmaceuticals LLC (827748190) Establishment Name Address ID/FEI Business Operations Gland Pharma Limited 918601238 ANALYSIS(70121-1002, 70121-1003) , LABEL(70121-1002, 70121-1003) , MANUFACTURE(70121-1002, 70121-1003) , PACK(70121-1002, 70121-1003)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.