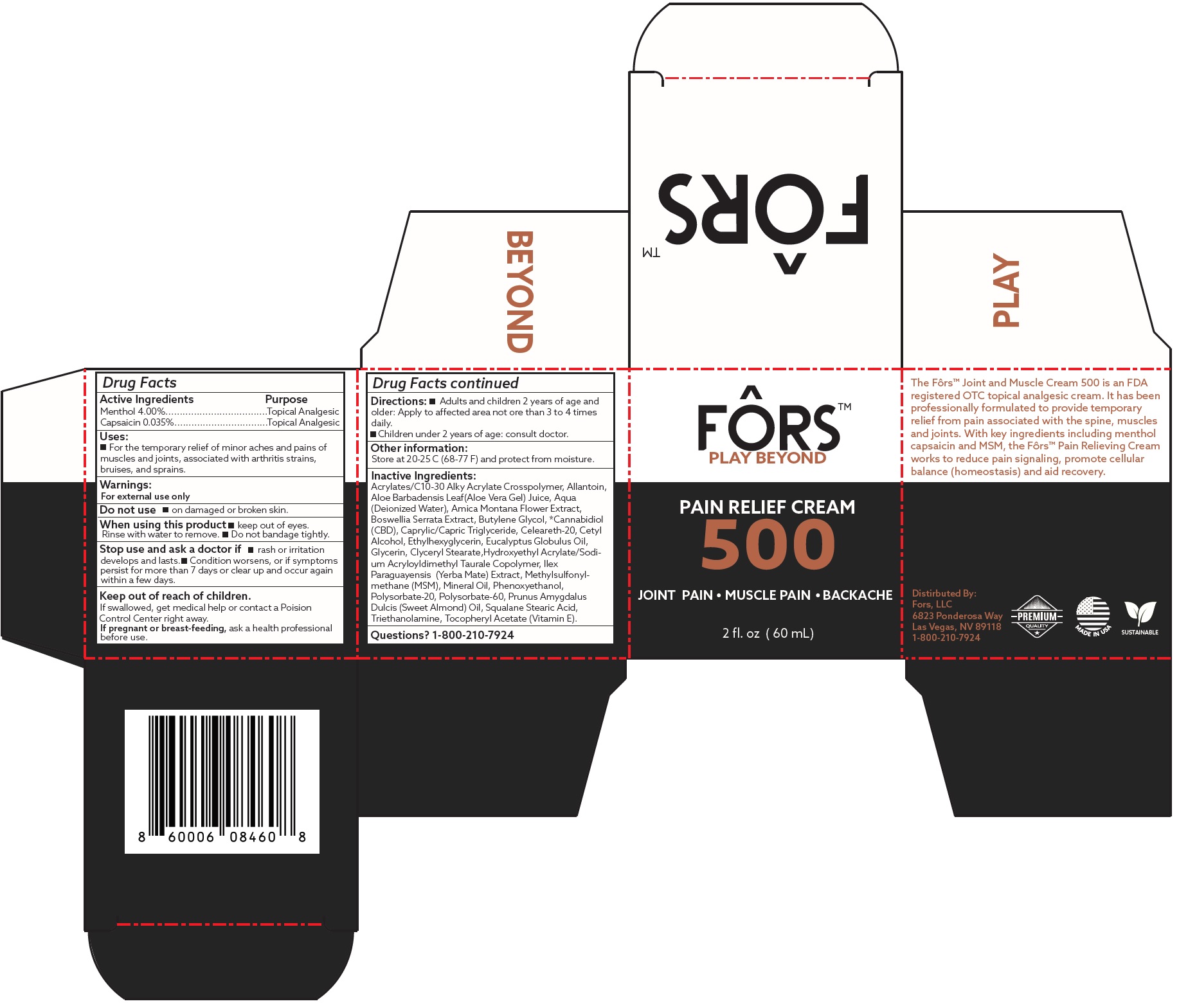
FORS PAIN RELIEF by FORS LLC FORS PAIN RELIEF CREAM
FORS PAIN RELIEF by
Drug Labeling and Warnings
FORS PAIN RELIEF by is a Otc medication manufactured, distributed, or labeled by FORS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FORS PAIN RELIEF- menthol, capsaicin cream
FORS LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
FORS PAIN RELIEF CREAM
Uses:
- For the temporary relief of minor aches and pains of muscles and joints, associated with arthritis strains, bruises, and sprains.
Warnings:
For external use only
Stop use and ask a doctor if
- rash or irritation develops and lasts.
- Condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days.
Directions:
- Adults and children 2 years of age and older: Apply to affected area not ore than 3 to 4 times daily.
- Children under 2 years of age: consult doctor.
Inactive Ingredients:
Acrylates/C10-30 Alky Acrylate Crosspolymer, Allantoin, Aloe Barbadensis Leaf(Aloe Vera Gel) Juice, Aqua (Deionized Water), Amica Montana Flower Extract, Boswellia Serrata Extract, Butylene Glycol, *Cannabidiol (CBD), Caprylic/Capric Triglyceride, Celeareth-20, Cetyl Alcohol, Ethylhexyglycerin, Eucalyptus Globulus Oil, Glycerin, Clyceryl Stearate,Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurale Copolymer, Ilex
Paraguayensis (Yerba Mate) Extract, Methylsulfonylmethane (MSM), Mineral Oil, Phenoxyethanol, Polysorbate-20, Polysorbate-60, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Squalane Stearic Acid, Triethanolamine, Tocopheryl Acetate (Vitamin E).
| FORS PAIN RELIEF
menthol, capsaicin cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - FORS LLC (117722650) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.