Nanz Pure by 1201258 Ontario Inc.O/A Nanz Pharma / 1201258 Ontario Inc. O/A Nanz Pharma Pure Calamine Lotion 19%
Nanz Pure by
Drug Labeling and Warnings
Nanz Pure by is a Otc medication manufactured, distributed, or labeled by 1201258 Ontario Inc.O/A Nanz Pharma, 1201258 Ontario Inc. O/A Nanz Pharma. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NANZ PURE- calamine lotion liquid
1201258 Ontario Inc.O/A Nanz Pharma
----------
Pure Calamine Lotion 19%
Uses:
For temporary relief of skin irritations and itching caused by sunburn, poison ivy, sumac and poison oak.
Caution and Warnings:
FOR EXTERNAL USE ONLY. Use only as directed.
When using this product, avoid contact with eyes. I contact occurs, rinse thoroughly with water.
Stop use and as/consult a doctor/physician/healthcare practitioner/healthcare provider/ healthcare professional if symptoms worsen or last for more than 7 days.
Do not use on broken skin.
KEEP OUT OF REACH OF CHILDREN.
In case of accidental ingestion, seek professional assistance or contact Poison Control Center immediately.
Directions:
Adults and children 2 years and older. Shake well before using. Cleanse the skin with soap and water and let dry before each use. Apply lotion to the affected area using cotton or soft cloth, as often as needed for comfort. Children under 6 months of age: consult a doctor before use.
| NANZ PURE
calamine lotion liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - 1201258 Ontario Inc.O/A Nanz Pharma (256906595) |
| Registrant - 1201258 Ontario Inc.O/A Nanz Pharma (256906595) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| 1201258 Ontario Inc. O/A Nanz Pharma | 256906595 | manufacture(83254-019) , pack(83254-019) , label(83254-019) | |
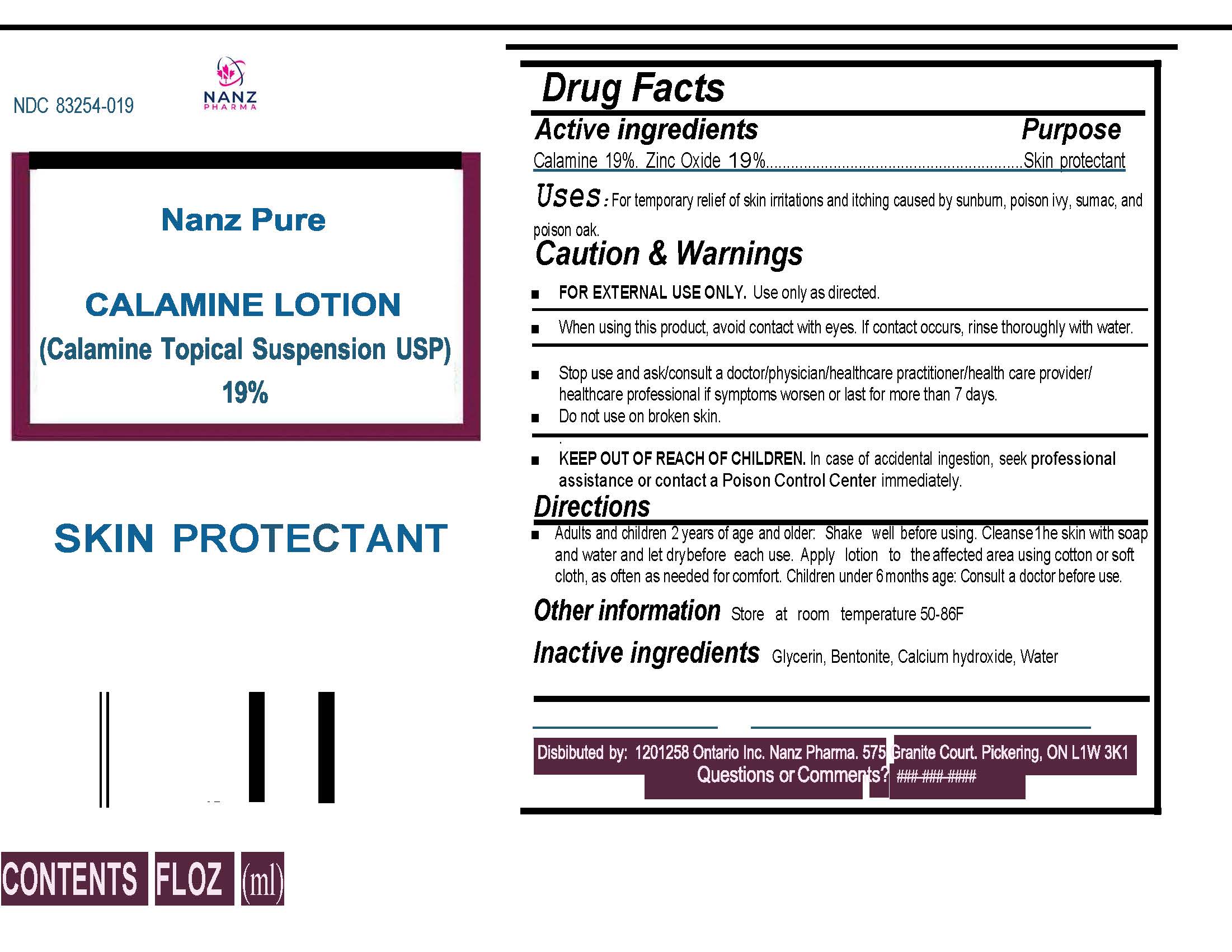 For all bottle sizes. A tentative Label
For all bottle sizes. A tentative Label