NITROGEN by Air Liquide Large Industries U.S. LP NITROGEN- nitrogen gas
NITROGEN by
Drug Labeling and Warnings
NITROGEN by is a Prescription medication manufactured, distributed, or labeled by Air Liquide Large Industries U.S. LP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Nitrogen N.F. COA
AIR LIQUIDE NITROGEN N.F. Form Number: 2A-ALL-QUA-0003-F
Revision: 0
Effective Date: 03/02/06
Page: 1/1
CERTIFICATE OF ANALYSIS
Air Liquide large Industries U.S. L.P. – Houston, TX 77056
PRODUCED BY AIR LIQUEFACTION
1.Location Street Address1 2. Carrier ________________
Street Address 2
City, State Zip
3. Lot Number ______________________ 4. Trailer No _____________
PRE-FILL REPORT FILL REPORT
5.Residual Assay 6. Odor 7. Trailer loaded with appropriate product per appropriate
(≤10 ppm O2) Detected loading procedure by:
_______ ppm Yes No Signature _________________________
________ ________ Date _____________________________
Initial Initial
ANALYSIS REPORT
Test required Method of Analysis Specifications Results
ASSAY AND 8.PARAMAGNETIC ___ 99.998% N2 Minimum* 10. ____%
IDENTIFICATION MICROFUEL CELL ___
ELECTROCHEMICAL ___
CELL 10 ppm O2 Maximum 11. _____ppm
9.Analyzer Tag Number
12.DETECTPR TUBE ___ 10 ppm CO Maximum 14. _____ppm
CARBON INFARED ANALYZER ___ Expiration Date of Tube 15. ______
MONOXIDE Lot Number of Tube 16. ______
13. Analyzer Tag Number
ODOR Organoleptic (Nasal) None 17. ______
N.F. applies only when used in a medical application or by medical customers properly registered with the FDA.
* Plus inerts
18. Analyzed By ________________________ Date ____________
19. Does this lot require additional testing? ___ NO ___ YES – attach page 2
20. SQCU Review/Release _______________________ Date ____________
MANDATORY FORM
User must assure that this revision of the form is current prior to use. Completed forms become permanent records subject to the record retention policy.

-
INGREDIENTS AND APPEARANCE
NITROGEN
nitrogen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11853-023 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGEN (UNII: N762921K75) (NITROGEN - UNII:N762921K75) NITROGEN 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11853-023-60 25000 L in 1 TANK; Type 0: Not a Combination Product 01/01/1960 2 NDC: 11853-023-61 50000 L in 1 TANK; Type 0: Not a Combination Product 01/01/1960 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205738 01/01/1960 Labeler - Air Liquide Large Industries U.S. LP (180015062) Registrant - Air Liquide Large Industries U.S. LP (180015062) Establishment Name Address ID/FEI Business Operations Air Liquide Large Industries U.S. LP 791294122 manufacture(11853-023) Establishment Name Address ID/FEI Business Operations Air Liquide Large Industries U.S. LP 832290014 manufacture(11853-023) Establishment Name Address ID/FEI Business Operations Air Liquide Large Industries U.S. LP 831993303 manufacture(11853-023) Establishment Name Address ID/FEI Business Operations Air Liquide Large Industries U.S. LP 047089305 manufacture(11853-023) Establishment Name Address ID/FEI Business Operations Air Liquide Large Industries U.S. LP 111765132 manufacture(11853-023) Establishment Name Address ID/FEI Business Operations Air Liquide Large Industries U.S. LP 117158467 manufacture(11853-023)
Trademark Results [NITROGEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
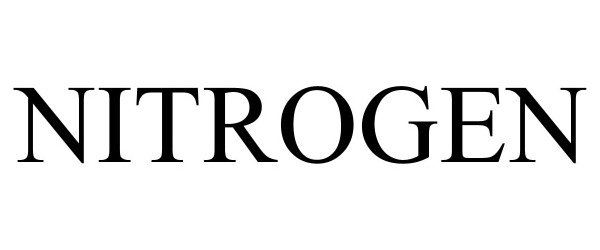 NITROGEN 98399875 not registered Live/Pending |
Intelligent Elephant 2024-02-09 |
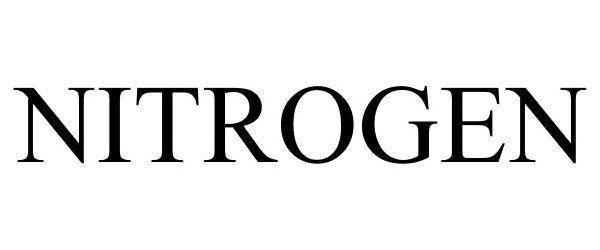 NITROGEN 97922386 not registered Live/Pending |
Riskalyze, Inc. 2023-05-05 |
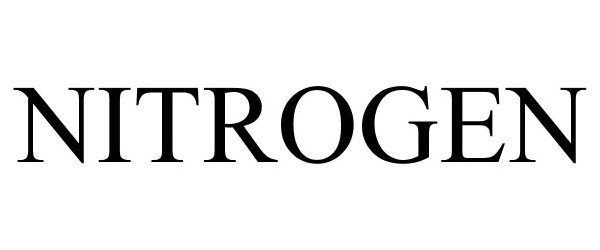 NITROGEN 87783527 not registered Live/Pending |
Raffles Investments (Proprietary) Limited 2018-02-04 |
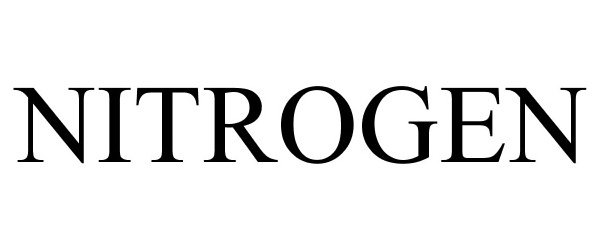 NITROGEN 87478016 not registered Dead/Abandoned |
NITROGEN INTERNATIONAL LTD. 2017-06-07 |
 NITROGEN 86224615 4839264 Live/Registered |
CYCLES ARGON-18 INC. 2014-03-18 |
 NITROGEN 78592715 3114002 Live/Registered |
JB INTERNATIONAL HOLDINGS LIMITED 2005-03-22 |
 NITROGEN 77961992 4527370 Live/Registered |
Huntsworth plc 2010-03-18 |
 NITROGEN 77449738 3592799 Live/Registered |
Jay-Y Enterprise Co., Inc. 2008-04-16 |
 NITROGEN 75759202 not registered Dead/Abandoned |
PROLAB NUTRTION, INC. 1999-07-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.