CHLOROQUINE PHOSPHATE tablet, coated
Chloroquine Phosphate by
Drug Labeling and Warnings
Chloroquine Phosphate by is a Prescription medication manufactured, distributed, or labeled by Avet Pharmaceuticals Inc., Suven Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
PATIENT PACKAGE INSERT
CHLOROQUINE PHOSPHATE TABLETS USP, 500 mg
DESCRIPTION
Chloroquine phosphate tablet, USP, is a 4-aminoquinoline compound for oral administration. It is a white, odorless, bitter tasting, crystalline substance, freely soluble in water.
Chloroquine phosphate tablet is an antimalarial and amebicidal drug.
Chemically, it is 7-chloro-4-[[4-(diethylamino)-1-methylbutyl]amino]quinoline phosphate (1:2) and has the following structural formula:
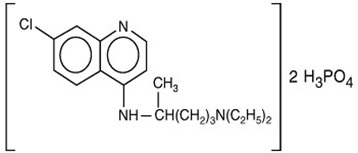
Each tablet contains 500 mg of chloroquine phosphate USP, equivalent to 300 mg chloroquine base.
CLINICAL PHARMACOLOGY
Chloroquine is rapidly and almost completely absorbed from the gastrointestinal tract, and only a small proportion of the administered dose is found in the stools. Approximately 55% of the drug in the plasma is bound to nondiffusible plasma constituents. Excretion of chloroquine is quite slow but is increased by acidification of the urine. Chloroquine is deposited in the tissues in considerable amounts. In animals, from 200 to 700 times the plasma concentration may be found in the liver, spleen, kidney, and lung; leukocytes also concentrate the drug. The brain and spinal cord, in contrast, contain only 10 to 30 times the amount present in plasma.
Chloroquine undergoes appreciable degradation in the body. The main metabolite is desethylchloroquine, which accounts for one fourth of the total material appearing in the urine; bisdesethylchloroquine, a carboxylic acid derivative, and other metabolic products as yet uncharacterized are found in small amounts. Slightly more than half of the urinary drug products can be accounted for as unchanged chloroquine.
Cardiac Electrophysiology
QTc interval prolongation was studied in a randomized, placebo-controlled parallel trial in 116 healthy subjects who received either chloroquine (1000 mg) alone or in combination with oral azithromycin (500 mg, 1000 mg, and 1500 mg once daily). Co-administration of azithromycin increased the QTc interval in a dose- and concentration- dependent manner. In comparison to chloroquine alone, the maximum mean (95% upper confidence bound) increases in QTcF were 5 ms, 7 (12) ms and 9 (14) ms with the co-administration of 500 mg, 1000 mg and 1500 mg azithromycin, respectively.
Microbiology
Mechanism of Action: Chloroquine, a 4-aminoquinoline, is an anti-protozoal agent. The precise mechanism by which chloroquine exhibits activity is not known. Chloroquine, may exert its effect against Plasmodiumspecies by concentrating in the acid vesicles of the parasite and by inhibiting polymerization of heme. It can also inhibit certain enzymes by its interaction with DNA.
Activity in Vitroand in Clinical Infections: Chloroquine is active against the erythrocytic forms of susceptible strains of Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax. Chloroquine is not active against the gametocytes and the exoerythrocytic forms including the hypnozoite stage ( P. vivaxand P. ovale) of the Plasmodiumparasites.
In vitro studies with Chloroquine demonstrated that it is active against the trophozoites of Entamoeba histolytica.
Drug Resistance: Resistance of Plasmodiumparasites to chloroquine is widespread (see INDICATIONS AND USAGE, Limitations of Use in Malaria and WARNINGS).
Plasmodiumparasites exhibiting reduced susceptibility to hydroxychloroquine also show reduced susceptibility to chloroquine.
Patients in whom chloroquine or hydroxychloroquine have failed to prevent or cure clinical malaria or parasitemia, or patients who acquired malaria in a geographic area where chloroquine resistance is known to occur should be treated with another form of antimalarial therapy (see WARNINGS and INDICATIONS AND USAGE, Limitations of Use).
INDICATIONS AND USAGE
Chloroquine phosphate tablets are indicated for the:
- Treatment of uncomplicated malaria due to susceptible strains of P. falciparum, P.malariae, P. ovale,and P.vivax.
- Prophylaxis of malaria in geographic areas where resistance to chloroquine is not present.
- Treatment of extraintestinal amebiasis.
Chloroquine phosphate tablets do not prevent relapses in patients with vivax or ovale malaria because it is not effective against exoerythrocytic forms of the parasites.
Limitations of Use in Malaria:
- Do not use Chloroquine phosphate tablets for the treatment of complicated malaria (high-grade parasitemia and/or complications e.g., cerebral malaria or acute renal failure).
- Do not use Chloroquine phosphate tablets for malaria prophylaxis in areas where chloroquine resistance occurs, Resistance to Chloroquine phosphate tablets is widespread in P. falciparum, and is reported in P.vivax(see WARNINGS).
- Concomitant therapy with an 8-aminoquinoline drug is necessary for treatment of the hypnozoite liver stage forms of P.vivaxand P.ovale(see DOSAGE AND ADMINISTRATION).
CONTRAINDICATIONS
Use of Chloroquine phosphate tablets for indications other than acute malaria is contraindicated in the presence of retinal or visual field changes of any etiology.
Use of Chloroquine phosphate tablets is contraindicated in patients with known hypersensitivity to 4- aminoquinoline compounds.
WARNINGS
Chloroquine-Resistant Malaria
Chloroquine phosphate tablets are not effective against chloroquine-or hydroxychloroquine-resistant strains of Plasmodiumspecies (see CLINICAL PHARMACOLOGY, Microbiology). Chloroquine resistance is widespread in P. falciparumand is reported in P. vivax. Before using chloroquine for prophylaxis, it should be ascertained whether chloroquine is appropriate for use in the region to be visited by the traveler. Information regarding the geographic areas where resistance to chloroquine occurs, is available at the Centers for Disease Control and Prevention (www.cdc.gov\malaria).
Patients infected with a resistant strain of plasmodia as shown by the fact that normally adequate doses have failed to prevent or cure clinical malaria or parasitemia should be treated with another form of antimalarial therapy.
Treatment of Exo-Erythocytic Forms of Malaria
Chloroquine does not treat the hypnozoite liver stage forms of Plasmodiumand will therefore not prevent relapses of malaria due to P. vivaxor P. ovale. Additional treatment with an anti-malarial agent active against these forms, such as an 8-aminoquinoline, is required for the treatment of infections witH P. vivaxand P. ovale.
Cardiac Effects including Cardiomyopathy and QT prolongation
Fatal and life-threatening cardiotoxicity, including cardiomyopathy and arrhythmias, have been reported with the use of chloroquine. (see ADVERSE REACTIONS and OVERDOSAGE). In multiple cases, endomyocardial biopsy showed association of the cardiomyopathy with phospholipidosis in the absence of inflammation, infiltration, or necrosis. Patients may present with ventricular hypertrophy and conduction disorders. ECG findings include atrioventricular, right or left bundle branch block. Chronic toxicity should be considered when conduction disorders (bundle branch block / atrio-ventricular heart block) are diagnosed.
QT interval prolongation, torsades de pointes, and ventricular arrhythmias have been reported with the use of chloroquine. The risk is greater if chloroquine is administered at high doses. Chloroquine should be used with caution in patients with cardiac disease, a history of ventricular arrhythmias, uncorrected hypokalemia and/or hypomagnesemia, or bradycardia (<50 bpm), and during concomitant administration with QT interval prolonging agents due to potential for QT interval prolongation (see WARNINGS, PRECAUTIONS, Drug Interactions, ADVERSE REACTIONS and OVERDOSAGE). Monitor for signs and symptoms of cardiotoxicity during chloroquine therapy. Discontinue chloroquine if cardiotoxicity is suspected or demonstrated by tissue biopsy.
Skeletal Muscle Myopathy and Neuropathy
Skeletal muscle myopathy or neuropathy leading to progressive weakness and atrophy of proximal muscle groups, and abnormal nerve conduction, have been reported. Muscle and nerve biopsies have shown associated phospholipidosis. Assess muscle strength and deep tendon reflexes periodically in patients on long-term therapy with chloroquine. Discontinue chloroquine if muscle or nerve toxicity is suspected or demonstrated by tissue biopsy.
Hypoglycemia
Chloroquine has been shown to cause severe hypoglycemia including loss of consciousness that could be life-threatening in patients treated with or without antidiabetic medications (see PRECAUTIONS, Drug Interactions). Patients treated with Chloroquine phosphate tablets should be warned about the risk of hypoglycemia and the associated clinical signs and symptoms. Patients presenting with clinical symptoms suggestive of hypoglycemia during treatment with chloroquine should have their blood glucose level checked and treatment reviewed as necessary.
Retinopathy 1
Irreversible retinal damage has been observed in some patients who had received chloroquine. Significant risk factors for retinal damage include daily doses of chloroquine phosphate greater than 2.3 mg/kg of actual body weight, durations of use greater than five years, subnormal glomerular filtration, use of some concomitant drug products such as tamoxifen citrate (see PRECAUTIONS), and concurrent macular disease.
A baseline ophthalmological examination should be performed within the first year of starting chloroquine phosphate tablets. The baseline exam should include: best corrected distance visual acuity (BCVA), an automated threshold visual field (VF) of the central 10 degrees (with retesting if an abnormality is noted), and spectral domain optical coherence tomography (SD-OCT).
For individuals with significant risk factors (daily dose of chloroquine phosphate greater than 2.3 mg/kg of actual body weight, subnormal glomerular filtration, use of tamoxifen citrate or concurrent macular disease) monitoring should include annual examinations which include BCVA, VF and SD-OCT. For individuals without significant risk factors, annual exams (including BCVA, VF and SD-OCT) can usually be deferred until five years of treatment.
In individuals of Asian descent, retinal toxicity may first be noticed outside the macula. In patients of Asian descent, it is recommended that visual field testing be performed in the central 24 degrees instead of the central 10 degrees.
It is recommended that chloroquine be discontinued if ocular toxicity is suspected and the patient should be closely observed given that retinal changes (and visual disturbances) may progress even after cessation of therapy.
Central Nervous System Effects
Acute extrapyramidal disorders may occur with chloroquine (see PRECAUTIONS, ADVERSE REACTIONS and OVERDOSAGE). These adverse reactions usually resolve after treatment discontinuation and/or symptomatic treatment.
Neuropsychiatric Reactions Including Suicidality
Suicidal behavior, suicidal ideation, and other neuropsychiatric adverse reactions have been reported in patients treated with chloroquine (see ADVERSE REACTIONS). Neuropsychiatric adverse reactions typically occur within the first month after the start of treatment with chloroquine and have been reported in patients with and without a prior history of psychiatric disorders. The risks and benefits of continued treatment with chloroquine should be assessed for patients who develop these symptoms. Given the long half-life of the drug, some patients may require several weeks off drug for symptoms to partially or fully abate.
Advise patients to contact their healthcare provider promptly if they experience new or worsening neuropsychiatric symptoms such as depression, suicidal thoughts or behavior, or mood changes
Renal Toxicity
Proteinuria with or without a moderate reduction in glomerular filtration rate have been reported with the use of chloroquine. Renal biopsy showed phospholipidosis without immune deposits, inflammation, and/or increased cellularity. Physicians should consider phospholipidosis as a possible cause of renal injury in patients with underlying connective tissue disorders who are receiving chloroquine. Discontinue chloroquine if renal toxicity is suspected or demonstrated by tissue biopsy.
Pediatric Accidental IngestionA number of fatalities have been reported following the accidental ingestion of chloroquine, sometimes in relatively small doses (0.75 g or 1 g chloroquine phosphate in one 3-year-old child). Patients should be strongly warned to keep Chloroquine phosphate tablets out of the reach of children because they are especially sensitive to the 4-aminoquinoline compounds (see OVERDOSAGE and ADVERSE REACTIONS).
Worsening of PsoriasisUse of Chloroquine phosphate tablets in patients with psoriasis may precipitate a severe attack of psoriasis. Chloroquine phosphate tablets should not be used in psoriasis unless the benefit to the patient outweighs the potential risks.
Risks Associated with Use in Porphyria
Administration of chloroquine to patients with porphyria may exacerbate porphyria. Avoid chloroquine in patients with porphyria. Hepatotoxicity Associated with Porphyria Cutanea Tarda Cases of hepatotoxicity have been reported when chloroquine was used in patients with porphyria cutanea tarda (PCT). Patients received dosages up to 500 mg per day. Most of the PCT-related cases presented with marked elevations in transaminases (>20 times the upper limit of the reference range) within days to a month of chloroquine initiation. Measure liver tests promptly in patients who report symptoms that may indicate liver injury, such as fatigue, rash, nausea, dark urine, or jaundice. In this clinical context, if the patient is found to have abnormal serum liver tests, interrupt chloroquine treatment and investigate further to establish the probable cause.
The safety and effectiveness of chloroquine for the treatment of PCT have not been established and chloroquine is not approved for this use.
Potential Carcinogenic Risk
Experimental data showed a potential risk of inducing gene mutations. There is insufficient evidence regarding the carcinogenicity of chloroquine in experimental animals (see PRECAUTIONS, Carcinogenesis, Mutagenesis, Impairment of Fertility). In humans, there are insufficient data to rule out an increased risk of cancer in patients receiving long-term treatment.
Usage in Pregnancy
In animal studies, embryo-fetal developmental toxicity was shown at doses ranging from 250 to 1500 mg/kg body weight; approximately 3 to 16 times the maximum recommended therapeutic dose based on a body surface area comparison (see PRECAUTIONS, Animal Pharmacology and/or Animal Toxicology). Preclinical data showed a potential risk of genotoxicity in some test systems (PRECAUTIONS, Carcinogenesis, Mutagenesis, Impairment of Fertility).
In humans, at recommended doses for prophylaxis and treatment of malaria, observational studies as well as a meta-analysis, including a small number of prospective studies with chloroquine exposure during pregnancy, have shown no increase in the rate of birth defects or spontaneous abortions.
The individual benefit-risk balance should be reviewed before prescribing chloroquine in pregnant women.
PRECAUTIONS
Hematological Effects/Laboratory Tests
Complete blood cell counts should be checked periodically if patients are given prolonged therapy (see ADVERSE REACTIONS).
Chloroquine may cause hemolysis in glucose-6 phosphate dehydrogenase (G-6-PD) deficiency. Blood monitoring may be needed as hemolytic anemia may occur, in particular in association with other drugs that cause hemolysis (see ADVERSE REACTIONS).
Auditory Effects
In patients with preexisting auditory damage, chloroquine should be administered with caution. In case of any defects in hearing, chloroquine should be immediately discontinued, and the patient closely observed (see ADVERSE REACTIONS).
Use in Patients with Hepatic Impairment
Since Chloroquine phosphate tablets are known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
Central Nervous System Effects
Chloroquine may increase the risk of convulsions in patients with a history of epilepsy.
Drug Interactions
Antacids and kaolin: Antacids and kaolin can reduce absorption of chloroquine; an interval of at least 4 hours between intake of these agents and chloroquine should be observed.
Cimetidine: Cimetidine can inhibit the metabolism of chloroquine, increasing its plasma level. Concomitant use of cimetidine should be avoided.
Insulin and other antidiabetic drugs: As chloroquine may enhance the effects of a hypoglycemic treatment, a decrease in doses of insulin or other antidiabetic drugs may be required.
Arrhythmogenic drugs: There may be an increased risk of inducing ventricular arrhythmias if chloroquine is used concomitantly with other arrhythmogenic drugs, such as amiodarone or moxifloxacin.
Ampicillin: In a study of healthy volunteers, chloroquine significantly reduced the bioavailability of ampicillin. An interval of at least two hours between intake of ampicillin and chloroquine should be observed.
Cyclosporine: After introduction of chloroquine (oral form), a sudden increase in serum cyclosporine level has been reported. Therefore, close monitoring of serum cyclosporine level is recommended and, if necessary, chloroquine should be discontinued.
Mefloquine: Co-administration of chloroquine and mefloquine may increase the risk of convulsions.
The blood concentrations of chloroquine and desethylchloroquine (the major metabolite of chloroquine, which also has antimalarial properties) were negatively associated with log antibody titers. Chloroquine taken in the dose recommended for malaria prophylaxis can reduce the antibody response to primary immunization with intradermal human diploid-cell rabies vaccine.
Praziquantel: In a single-dose interaction study, chloroquine has been reported to reduce the bioavailability of praziquantel.
Tamoxifen: Concomitant use of chloroquine with drugs known to induce retinal toxicity such as tamoxifen is not recommended (see WARNINGS).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
There is insufficient evidence regarding the carcinogenicity of chloroquine in experimental animals.
Mutagenesis
Chloroquine is reported in the literature to be a weak genotoxic agent which may elicit both gene mutations and chromosomal/DNA breaks. Mechanisms may involve DNA intercalation or induction of oxidative stress. The publications reported both positive and negative results in the in vitro reverse gene mutation assays using bacteria (Ames test) and in the in vivo studies using rodents (mouse bone marrow cell sister chromatid exchange, mouse bone marrow cell chromosome abnormality, and rat DNA strand breaks in multiple organs when animals were dosed by intraperitoneal route). Those chromosomal effects were not observed when Chloroquine was administered in animals orally (i.e. the therapeutic route of administration). Altogether those cellular and rodent data show evidence of genotoxic potential but discrepancies have been noted between published data.
Fertility
A study in male rats after 30 days of oral treatment at 5 mg/day of chloroquine showed a decrease in testosterone levels, weight of testes, epididymis, seminal vesicles, and prostate. Untreated female rats had reduced number of fetuses after mating with males that received intraperitoneal injections of 10 mg/kg chloroquine for 14 days.
Animal Pharmacology and/or Animal Toxicology
Based on non-Good Laboratory Practice literature reports, chloroquine causes malformations in rats, resulting in a fetal mortality rate of 25% after administration at doses between 250 and 1500 mg/kg (approximately 3 to 16 times the maximum recommended therapeutic dose of 900 mg of chloroquine base, based on a body surface area comparison), showing ocular malformations in 45% of fetuses in the 1000 mg/kg group (approximately 11 times the maximum recommended therapeutic dose based on a body surface area comparison). Autoradiographic studies have shown that when administered at the start or the end of gestation, Chloroquine accumulates in the eyes and ears.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from chloroquine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential clinical benefit of the drug to the mother.
The excretion of chloroquine and the major metabolite, desethylchloroquine, in breast milk was investigated in eleven lactating mothers following a single oral dose of chloroquine (600 mg base). The maximum daily dose of the drug that the infant can receive from breastfeeding was about 0.7% of the maternal start dose of the drug in malaria chemotherapy. Separate chemoprophylaxis for the infant is required (see DOSAGE AND ADMINISTRATION).
Geriatric Use
Clinical studies of Chloroquine phosphate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
ADVERSE REACTIONS
The following adverse reactions have been identified during post-approval use of chloroquine or other 4-aminoqunoline compounds. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Ocular disorders:Maculopathy and macular degeneration have been reported and may be irreversible. Irreversible retinopathy with retinal pigmentation changes (bull’s eye appearance) and visual field defects (paracentral scotomas) in patients receiving long-term or high-dosage 4-aminoquinoline therapy have been reported (see WARNINGS). Visual disturbances (blurring of vision and difficulty of focusing or accommodation); nyctalopia; scotomatous vision with field defects of paracentral, pericentral ring types, and typically temporal scotomas (e.g., difficulty in reading with words tending to disappear, seeing half an object, misty vision, and fog before the eyes) have been reported. Reversible corneal opacities have also been reported.
Immune system disorders:Urticaria, anaphylactic reaction including angioedema.
Ear and labyrinth disorders:Nerve type deafness; tinnitus, reduced hearing in patients with preexisting auditory damage.
Musculoskeletal and connective tissue-disorders:Sensorimotor disorders, skeletal muscle myopathy or neuromyopathy leading to progressive weakness and atrophy of proximal muscle groups, depression of tendon reflexes and abnormal nerve conduction.
Gastrointestinal disorders:Hepatitis, increased liver enzymes, anorexia, nausea, vomiting, diarrhea, abdominal cramps.
Skin and subcutaneous tissue disorders: Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, exfoliative dermatitis. Pleomorphic skin eruptions, skin and mucosal pigmentary changes; lichen planus-like eruptions, pruritus,; drug rash with eosinophilia and systemic symptoms (DRESS syndrome); photosensitivity and hair loss and bleaching of hair pigment.
Blood and lymphatic system disorders:Pancytopenia, aplastic anemia, reversible agranulocytosis, thrombocytopenia and neutropenia. Hemolytic anemia in G6PD deficient patients (see PRECAUTIONS).
Nervous system disorders:Convulsions, mild and transient headache, polyneuropathy, acute extrapyramidal disorders (such as dystonia, dyskinesia, tongue protrusion, torticollis) (see WARNINGS and OVERDOSAGE).
Neuropsychiatric disorders:Neuropsychiatric changes including psychosis, delirium, anxiety, agitation, insomnia, confusion, hallucinations, personality changes, depression, suicidal behavior, suicidal ideation, affect/emotional lability, irritability, nervousness, nightmares/sleep terrors, depression, delusions, paranoia, and mania.
Cardiac disorders:Hypotension, electrocardiographic changes (particularly, inversion or depression of the T-wave with widening of the QRS complex), and cardiomyopathy (which may result in cardiac failure and in some cases a fatal outcome).
Cardiac arrhythmias, conduction disorders such as bundle branch block / atrio-ventricular block, QT interval prolongation, torsade de pointes, ventricular tachycardia and ventricular fibrillation have been reported with therapeutic doses of chloroquine as well as with overdose. The risk is greater if chloroquine is administered at high doses. Fatal cases have been reported (see WARNINGS, Cardiac Effects and OVERDOSAGE).
Metabolic and Nutritional disorders:Hypoglycemia (see WARNINGS).
To report SUSPECTED ADVERSE REACTIONS, contact Avet Pharmaceuticals at 1-866-901-DRUG (3784), or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Signs and Symptoms:Chloroquine is very rapidly and completely absorbed after ingestion. Toxic doses of chloroquine can be fatal. As little as 1 g may be fatal in children. Toxic symptoms can occur within minutes. The symptoms of overdosage may include nausea, vomiting, headache, drowsiness, visual disturbances, cardiovascular collapse, convulsions, hypokalemia, rhythm and conduction disorders including QT prolongation, torsades de pointes, ventricular tachycardia and ventricular fibrillation, followed by sudden potentially fatal respiratory and cardiac arrest. Immediate medical attention is required, as these effects may appear shortly after the overdose. Cases of extrapyramidal disorders have also been reported in the context of chloroquine overdose (see WARNINGS and ADVERSE REACTIONS).
Treatment:Treatment is symptomatic and must be prompt with immediate evacuation of the stomach by emesis or gastric lavage followed by treatment with activated charcoal. Chloroquine overdose is a life- threatening emergency and should be managed with cardio-respiratory and hemodynamic support, monitoring of potassium along with management of arrhythmias and convulsions, as necessary. A patient who survives the acute phase and is asymptomatic should be closely observed until all clinical features of toxicity resolve.
DOSAGE & ADMINISTRATION
The dosage of chloroquine phosphate is often expressed in terms of equivalent chloroquine base. Each 500 mg tablet of Chloroquine phosphate contains the equivalent of 300 mg chloroquine base. In infants and children the dosage is preferably calculated by body weight.
Prophylaxis against chloroquine-sensitive Plasmodiumspecies
Adult Dose:The dosage for prophylaxis is 500 mg (= 300 mg base) administered once per week on exactly the same day of each week.
Pediatric Dose:The dosage for prophylaxis is 5 mg calculated as base, per kg of body weight, administered once per week on exactly the same day of each week. The pediatric dose should never exceed the adult dose regardless of weight.
If circumstances permit, suppressive therapy should begin two weeks prior to exposure. However, failing this in adults, an initial double (loading) dose of 1 g (= 600 mg base), or in children 10 mg base/kg may be taken in two divided doses, six hours apart. The suppressive therapy should be continued for eight weeks after leaving the endemic area.
Treatment of uncomplicated malaria due to chloroquine-sensitive Plasmodiumspecies
Adults:An initial dose of 1 g salt (= 600 mg base) followed by an additional 500 mg (= 300 mg base) after six to eight hours and a single dose of 500 mg (= 300 mg base) on each of two consecutive days. This represents a total dose of 2.5 g chloroquine phosphate or 1.5 g base in three days.
Infants and Children:In infants and children, the recommended dose is 10 mg base/kg followed by 5 mg based/kg at 6, 24 and 36 hours (total dose 25 mg based/kg). The pediatric dose should never exceed the adult dose regardless of weight.
The dosage for adults of low body weight and for infants and children should be determined as follows:
First dose: 10 mg base per kg (but not exceeding a single dose of 600 mg base).
Second dose: (6 hours after first dose) 5 mg base per kg (but not exceeding a single dose of 300 mg base).
Third dose: (24 hours after first dose) 5 mg base per kg.
Fourth dose: (36 hours after first dose) 5 mg base per kg.
P. vivaxand P. ovale: Concomitant therapy with an 8-aminoquinoline compound is necessary for treatment of the hypnozoite liver stage forms of the parasites.
Extraintestinal Amebiasis:Adults Dosage: 1 g salt (600 mg base) daily for two days, followed by 500 mg (300 mg base) daily for at least two to three weeks. Treatment is usually combined with an effective intestinal amebicide.
Geriatric Use
See PRECAUTIONS, Geriatric Use.
HOW SUPPLIED
Chloroquine phosphate tablets, USP contains 500 mg chloroquine phosphate USP, equivalent to 300 mg of chloroquine base
Bottle of 25 (NDC: 23155-850-74)
White, round, biconvex film-coated tablets, with debossing of ‘CP 500’ on one side and plain surface on the other side.
Dispense in tight, light-resistant container as defined in the USP/NF.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
REFERENCES
- Marmor MF, Kellner U, and Lai TY, et al. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016. Doi10.1016./jophtha.2016.01.058
Rx Only
Manufactured by:Suven Pharmaceuticals Limited
Plot No’s.: 262 to 271, IDA Pashamylaram,
Sangareddy District 502307, Telangana, India
ML No: 24/MD/AP/2009/F/CC
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.91.DRUG (3784)
Avet Pharma®
Lb50395-3-01
Revised: 05/2023
-
25 Ct Bottle Label
NDC: 23155-850-74
Chloroquine Phosphate
Tablets, USP
500 mg*
Rx Only 25 Tablets
For use in Malaria and
Extraintestinal Amebiasis Only
:b50395-1-00
Rev. 01/2023Avet Pharma ®
*Each tablet contains :
Chloroquine phosphate, USP..... 500 mg
(Equivalent to 300 mg Chloroquine base)
USUAL ADULT DOSAGE :See accompanying product literature for
complete information.
Dispensein a tight, light resistant container as defined in the USP
using a child-resistant closure.
Store at 20o to 25oC (68o to 77oF); excursions permitted to
15o to 30oC (59o to 86oF) [see USP Controlled Room
Temperature]. Protect from light and moisture.
WARNING : Physicians should completely familiarize
themselves with the complete contents of the product
literature, before prescribing chloroquine phosphate.
KEEP OUT OF THE REACH OF CHILDREN
Manufacured by: Suven Pharmaceuticals Limited,
Pashamylaram, Telangana 502307, India
ML No: 24/MD/AP/2009/F/CC
Manufactured for: Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)
N3 23155 85074 1 
-
INGREDIENTS AND APPEARANCE
CHLOROQUINE PHOSPHATE
chloroquine phosphate tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 23155-850 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROQUINE PHOSPHATE (UNII: 6E17K3343P) (CHLOROQUINE - UNII:886U3H6UFF) CHLOROQUINE PHOSPHATE 500 mg Product Characteristics Color white Score no score Shape ROUND (bi-convex with "CP 500") Size 13mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 23155-850-74 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/20/2023 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214756 03/20/2023 Labeler - Avet Pharmaceuticals Inc. (780779901) Registrant - Suven Pharmaceuticals Limited (861468675) Establishment Name Address ID/FEI Business Operations Suven Pharmaceuticals Limited 677604288 pack(23155-850) , analysis(23155-850) , api manufacture(23155-850) , label(23155-850) , manufacture(23155-850)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.