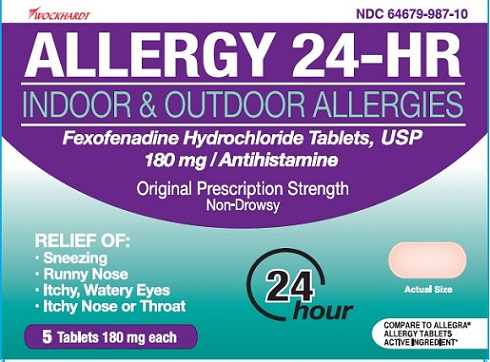
ALLERGY 24-HR- fexofenadine hydrochloride tablet, film coated
ALLERGY 24-HR by
Drug Labeling and Warnings
ALLERGY 24-HR by is a Otc medication manufactured, distributed, or labeled by Wockhardt USA LLC., Wockhardt Limited, Patheon Puerto Rico, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- OTC - ACTIVE INGREDIENT SECTION
- OTC - PURPOSE SECTION
- USAGE
-
WARNINGS
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have kidney disease. Your doctor should determine if you need a different dose.
When using this product
● do not take more than directed
● do not take at the same time as aluminum or magnesium antacids
● do not take with fruit juices (see Directions)
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding, ask a health professional before use.
- OTC - KEEP OUT OF REACH OF CHILDREN
-
DOSAGE AND ADMINISTRATION
adults and children 12 years of age and over
take two 30 mg tablets with water every 12 hours;
do not take more than 4 tablets in 24 hours
children 6 to under 12 years of age
take one 30 mg tablet with water every 12 hours;
do not take more than 2 tablets in 24 hours
children under 6 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
adults and children 12 years of age and over
take one 60 mg tablet with water every 12 hours;
do not take more than 2 tablets in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
adults and children 12 years of age and over
take one 180 mg tablet with water once a day;
do not take more than 1 tablet in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
- Other information
-
INACTIVE INGREDIENT
colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone K-30, talc, titanium dioxide
For 60 mg
colloidal silicon dioxide, croscarmellose sodium, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone K-30, talc, titanium dioxide
For 180 mg
colloidal silicon dioxide, croscarmellose sodium, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone K-30, talc, titanium dioxide
- Questions or comments?
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY 24-HR
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64679-744 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape ROUND Size 7mm Flavor Imprint Code W;30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64679-744-09 1 in 1 CARTON 02/08/2012 1 NDC: 64679-744-08 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079112 02/08/2012 ALLERGY 24-HR
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64679-982 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN (light peach to peach) Score no score Shape CAPSULE Size 11mm Flavor Imprint Code W982 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64679-982-09 2 in 1 CARTON 02/08/2012 1 NDC: 64679-982-08 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079112 02/08/2012 ALLERGY 24-HR
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64679-987 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN (light peach to peach) Score no score Shape CAPSULE Size 17mm Flavor Imprint Code W987 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64679-987-10 1 in 1 CARTON 02/08/2012 1 NDC: 64679-987-09 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 64679-987-11 3 in 1 CARTON 02/08/2012 2 NDC: 64679-987-09 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 64679-987-05 2000 in 1 POUCH; Type 0: Not a Combination Product 02/08/2012 4 NDC: 64679-987-20 1 in 1 CARTON 02/08/2012 4 NDC: 64679-987-12 30 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC: 64679-987-24 1 in 1 CARTON 02/08/2012 5 NDC: 64679-987-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC: 64679-987-14 2 in 1 CARTON 02/08/2012 6 NDC: 64679-987-13 45 in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC: 64679-987-17 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/08/2012 8 NDC: 64679-987-22 1 in 1 CARTON 02/08/2012 8 NDC: 64679-987-13 45 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079112 02/08/2012 Labeler - Wockhardt USA LLC. (170508365) Registrant - Wockhardt Limited (650069115) Establishment Name Address ID/FEI Business Operations Wockhardt Limited 676257570 ANALYSIS(64679-744, 64679-982, 64679-987) , MANUFACTURE(64679-744, 64679-982, 64679-987) , LABEL(64679-744, 64679-982, 64679-987) , PACK(64679-744, 64679-982, 64679-987) Establishment Name Address ID/FEI Business Operations Patheon Puerto Rico, Inc. 143814544 ANALYSIS(64679-982, 64679-987) , LABEL(64679-982, 64679-987) , MANUFACTURE(64679-982, 64679-987) , PACK(64679-982, 64679-987)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.