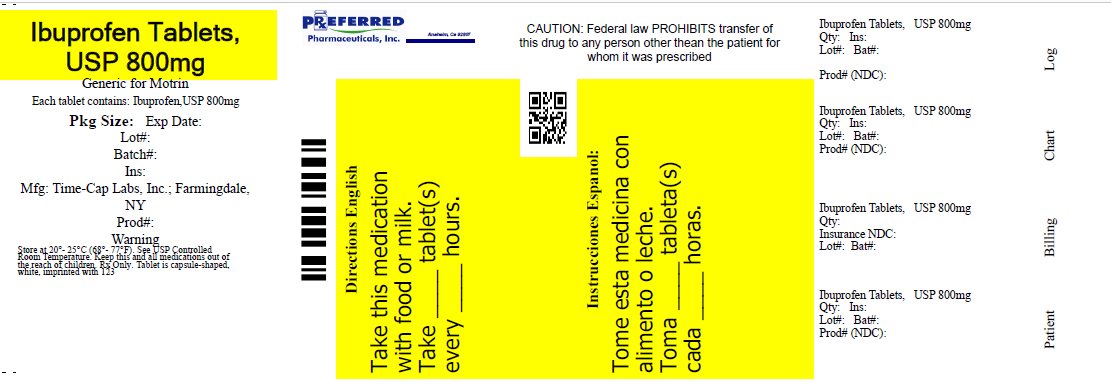
IBUPROFEN 400 MG - 600 MG AND 800 MG TABLETS
IBUPROFEN by
Drug Labeling and Warnings
IBUPROFEN by is a Prescription medication manufactured, distributed, or labeled by Preferred Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
IBUPROFEN- ibuprofen tablet, film coated
Preferred Pharmaceuticals Inc.
----------
IBUPROFEN 400 MG - 600 MG AND 800 MG TABLETS
800 mg (white to off-white, capsule shaped, biconvex, film-coated tablets debossed with ‘123’ on one side and plain on other side)
- Bottles of 14 NDC: 68788-7079-4
Repackaged By: Preferred Pharmaceuticals Inc.
| IBUPROFEN
ibuprofen tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Preferred Pharmaceuticals Inc. (791119022) |
| Registrant - Preferred Pharmaceuticals Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals Inc. | 791119022 | REPACK(68788-7079) | |
Revised: 7/2023
Document Id: d8b1b4f8-7e34-4f45-be18-d2430ebe934f
Set id: f4b778cf-0741-46b3-ace9-7ac8d0695e31
Version: 4
Effective Time: 20230713