FA Hand Sanitizer Liquid 70 by HLB CO.,LTD_Healthcare
FA Hand Sanitizer Liquid 70 by
Drug Labeling and Warnings
FA Hand Sanitizer Liquid 70 by is a Otc medication manufactured, distributed, or labeled by HLB CO.,LTD_Healthcare. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
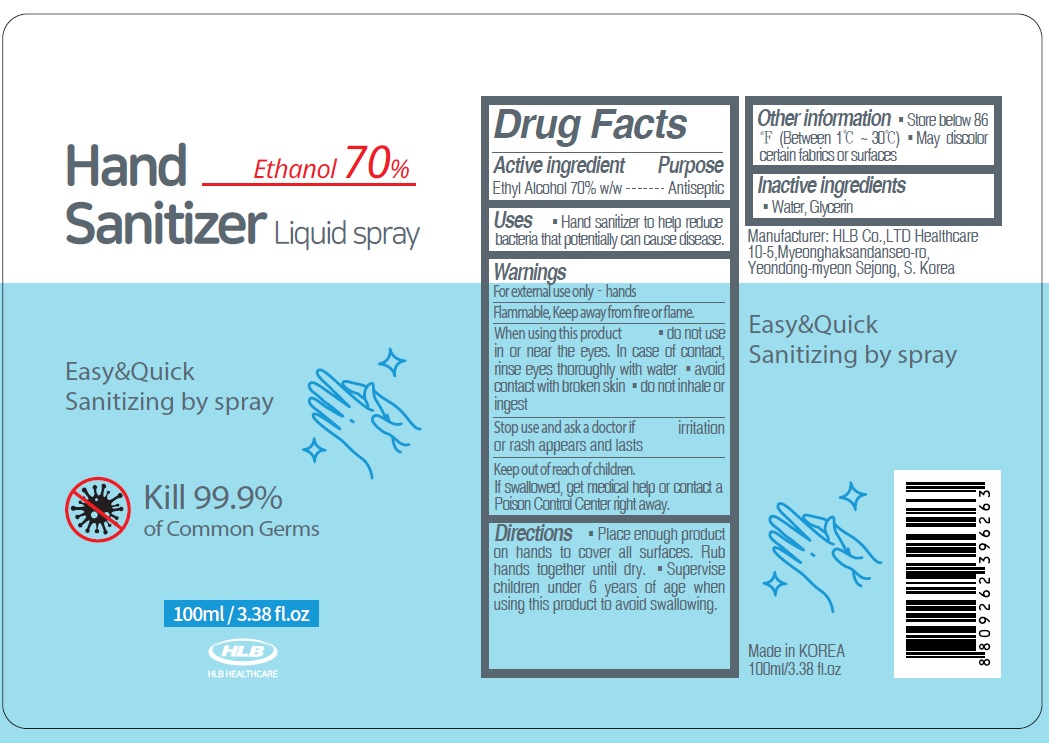
FA HAND SANITIZER LIQUID 70- alcohol spray
HLB CO.,LTD_Healthcare
----------
WARNINGS
Warnings:
For external use only. Flammable. Keep away from heat or flame
--------------------------------------------------------------------------------------------------------
When using this product ■ do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water ■ avoid contact with broken skin ■ do not inhale or ingest
--------------------------------------------------------------------------------------------------------
Stop use and ask a doctor if irritation or rash appears and lasts
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Directions
■ Place enough product on hands to cover all surfaces. Rub hands together until dry.
■ Supervise children under 6 years of age when using this product to avoid swallowing.
| FA HAND SANITIZER LIQUID 70
alcohol spray |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - HLB CO.,LTD_Healthcare (987587913) |
| Registrant - HLB CO.,LTD_Healthcare (987587913) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HLB CO.,LTD_Healthcare | 987587913 | manufacture(74932-400) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.