ONSIOR- robenacoxib injection
Onsior by
Drug Labeling and Warnings
Onsior by is a Animal medication manufactured, distributed, or labeled by Elanco US Inc,. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Caution:
-
Description:
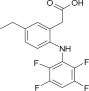
ONSIOR (robenacoxib) injection is a non-narcotic, non-steroidal anti-inflammatory drug
(NSAID) of the coxib class. Each mL of this clear sterile solution for injection contains
20 mg robenacoxib, 350 mg polyethylene glycol 400, 120 mg ethanol, 100 mg poloxamer 188, 1 mg sodium metabisulfite; citric acid and sodium hydroxide are used for pH adjustment. The molecular weight of robenacoxib is 327.28. The empirical formula is C16-H13-F4-NO2. Robenacoxib is [5-Ethyl-2-(2,3,5,6-tetrafluoro-phenylamino)-phenyl]acetic acid. The structural formula is:
- Indication:
-
Dosage and Administration:
Carefully consider the potential benefits and risks of ONSIOR and other treatment options before deciding to use ONSIOR (robenacoxib) injection. Use the lowest effective dose for the shortest duration consistent with individual response. It is recommended as good aseptic practice to clean stopper surface with isopropyl alcohol before and after each insertion of a needle.
The dose of ONSIOR (robenacoxib) injection is 0.91 mg/lb (2 mg/kg) subcutaneously once daily, for a maximum of 3 days.
Dosage Directions: For subcutaneous injection in cats ≥ 4 months of age; for up to a maximum of 3 days. To ensure accuracy of dosing, the use of a 1 mL graduated syringe is recommended. The first dose should be administered approximately 30 minutes prior to surgery, at the same time as the pre-anesthetic agents are given.
Subsequent doses can be given via subcutaneous injection, or interchanged with the oral tablet in cats ≥ 5.5 lbs and ≥ 4 months of age, for a maximum of 3 total ONSIOR doses over 3 days, not to exceed one dose per day (see Animal Safety and ONSIOR tablet product insert).
Note: In cats, the dose of ONSIOR tablets and ONSIOR injection are different.
- Contraindications:
-
Warnings:
Not for use in humans. Keep this and all medications out of reach of children.
Consult a physician in case of accidental ingestion or injection by humans.
For subcutaneous use in cats and dogs (see reverse side for instructions for use in dogs).
All cats should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory tests should be conducted to establish hematological and serum biochemical baseline data prior to administration of an NSAID.
Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions and Animal Safety).
Do not administer ONSIOR injection or tablets in conjunction with any other oral or injectable NSAID or corticosteroid.
-
Precautions:
When using NSAIDS such as ONSIOR, the use of fluid therapy during surgery is recommended to decrease potential renal complications (see Adverse Reactions, Post-Approval Experience).
Appetite should be monitored in cats receiving ONSIOR.
Stop administration of ONSIOR if appetite decreases or if the cat becomes lethargic.
Inflammation at the injection site may be noted.
The use of ONSIOR has not been evaluated in cats younger than 4 months of age and less than 5.5 lbs, cats used for breeding, or in pregnant or lactating cats. Long-term use of the injectable has not been studied. Safety has not been demonstrated for IV or IM administration.
The use of ONSIOR in cats with cardiac disease has not been studied. ONSIOR has been shown to prolong the QT interval.
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Cats that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Anesthetic drugs may affect renal perfusion; approach concomitant use of anesthetics and NSAIDs cautiously.
Appropriate monitoring procedures (including ECG, blood pressure, and temperature regulation) should be employed during all surgical procedures. The use of parenteral fluids during surgery is recommended to decrease potential renal complications when using NSAIDs perioperatively.
If additional pain medication is needed after a daily dose of ONSIOR, a non-NSAID/ non-corticosteroid class of analgesic may be necessary. Concurrent administration of potentially nephrotoxic drugs should be carefully approached and monitored. NSAIDs may inhibit prostaglandins which maintain normal homeostatic function. Such antiprostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. NSAIDs possess the potential to produce gastrointestinal ulcerations and/or gastrointestinal perforations. Do not use ONSIOR concomitantly with other anti-inflammatory drugs, such as NSAIDs or corticosteroids. Consider appropriate washout times when switching from one NSAID to another or when switching from corticosteroid use to NSAID use. ONSIOR injection and ONSIOR tablets are safe to use interchangeably when given once a day for a maximum of 3 days in cats ≥ 4 months of age and ≥ 5.5 lbs. Note: In cats, the dose of ONSIOR tablets and ONSIOR injection are different.
The use of concomitantly protein-bound drugs with ONSIOR has not been studied in cats. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications. The influence of concomitant drugs that may inhibit metabolism of ONSIOR has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy. Concurrent medications used during the field study with ONSIOR included parasiticides, anesthetics, pre-anesthetic medications, and antibiotics.
The effect of cyclo-oxygenase inhibition and the potential for thromboembolic occurrence or a hypercoagulable state has not been evaluated. It is unknown whether cats with a history of hypersensitivity to ß lactam drugs will exhibit hypersensitivity to ONSIOR. Robenacoxib is poorly soluble in water and in acid solutions readily degrades to form γ-lactam. In cats, lactam is a metabolite of robenacoxib. Additionally, lactam is a degradation product that increases over the shelf-life of the solution. Neurologic signs have been associated with the use of ß lactam drugs; it is unknown if the lactam in robenacoxib may cause similar neurologic signs (see Animal Safety). Occurrences of skin lesions/urticaria, seizures, ataxia, and nystagmus have been associated with the use of ONSIOR.
Robenacoxib may prolong the QT interval; the associated risk of developing ventricular arrhythmia is unknown. The use of robenacoxib with other drugs shown to prolong the QT interval is not recommended. Commonly used drugs that prolong the QT interval include antihistamines, prokinetic, and certain anesthetic drugs. ONSIOR (robenacoxib) injection contains sodium metabisulfite. ONSIOR (robenacoxib) injection should not be used in patients with sulfite hypersensitivity. As a class, it is unknown if the use of NSAIDs in asthmatic cats can cause acute asthmatic exacerbations, as seen in some humans.
-
Adverse Reactions:
The controlled field study (see Effectiveness) included a total of 349 healthy male and female cats in the field safety analysis. ONSIOR (robenacoxib) injection treated cats represented 7 breeds, 4 months to 7 years old, weighing 5.5-13.2 lbs. The following table shows the number of cats exhibiting each observation.
Adverse Reactions in the Postoperative Pain Field Study *Cats may have experienced more than one type or occurrence of an event during the study.
**One ONSIOR cat suffered an anesthetic-related death.
***Semiconscious cat fully recovered.
Adverse Reaction* ONSIOR N = 174** Control
(0.9% physiologic saline) N = 175Incision site infection, dehiscence 9 0 Increased incision site bleeding 6 4 Vomiting 5 0 Decreased appetite 4 3 Lethargy (after day of surgery) 4 2 UTI 2 0 Coughing 1 0 Fever 1 0 Semiconscious*** 1 0 Soft stool, diarrhea 0 2 The most commonly reported adverse reactions in cats treated with ONSIOR
(robenacoxib) injection were infected incision sites, increased incision site bleeding, vomiting, inappetence, and lethargy. Changes in the clinical pathology values were not considered clinically significant.
An additional study was conducted in client-owned cats to evaluate ONSIOR
(robenacoxib) injection at a dose of 2 mg/kg administered once daily for the control of postoperative pain and inflammation associated with an onychectomy (forelimbs only) and OVH or castration. The study was a masked, negative controlled, multi-center field study in which 187 cats were enrolled in a 2:1 ratio into two groups (127 cats treated with robenacoxib injection and 60 cats treated with saline).
Adverse Reactions from Additional Field Study *Cats may have experienced more than one type or occurrence of an event during the study.
Adverse Reaction* ONSIOR N = 127 Control
(0.9% physiologic saline N = 60Surgical or venipuncture site bleeding 9 3 Leukocytosis 9 2 Fever 8 11 Lethargy
(after day of surgery)7 0 Vomiting 6 0 Anorexia/ decreased appetite 6 3 Infected/drainage surgical site(s), infection 5 2 Dehiscence 1 0 Diarrhea 1 3 Bloody stool 1 0 Hypersalivation 1 2 UTI 0 1 The mean WBC count in the ONSIOR group was 13.8 ± 5.64 103/μL (ranging from
4.4 – 36.8) and the mean WBC count in the control group was 12.7 ± 5.41 103/μL (with values ranging from 2.9 – 30.1) at the end of the study. There were 6 ONSIOR cases with elevated ALT post-treatment compared to 1 control case. There were 4 ONSIOR cases with elevated AST post-treatment compared to 1 control case.
The most commonly reported adverse reactions in cats treated with ONSIOR were increased incision site bleeding, leukocytosis, fever, inappetence, infected incision sites, vomiting, and lethargy.
The results of the field study demonstrate that ONSIOR injection, when administered for a maximum of 3 days, is well-tolerated for the control of postoperative pain associated with onychectomy, ovariohysterectomy, and castration in cats.
-
Post Approval Experience (2015):
The following adverse events are based on voluntary, post approval reporting. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The signs reported are listed in decreasing order of reporting frequency for ONSIOR tablets in cats:
Anorexia, depression/lethargy, vomiting, elevated BUN, elevated creatinine, renal insufficiency/ failure, diarrhea, weight loss, dehydration.
In some cases, death has been reported. Some of these cases involved patients that developed renal failure/renal insufficiency.
To report suspected adverse drug events and for technical assistance, contact Elanco US Inc. at 1-888-545-5973.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/ SafetyHealth.
-
Information for Cat Owners:
ONSIOR, like other drugs of its class, is not free from adverse reactions. Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance.
Adverse reactions may include vomiting, diarrhea, decreased appetite, dark or tarry stools, increased water consumption, increased urination, anemia, yellowing of gums, skin or white of the eye due to jaundice, lethargy, incoordination, seizure, or behavioral changes. Serious adverse reactions associated with this drug class can occur without warning and result in death (see Warnings and Adverse Reactions). Owners should be advised to contact their veterinarian immediately if signs of intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated.
-
Clinical Pharmacology:
In an inflammation model in cats, robenacoxib had analgesic, anti-inflammatory and anti-pyretic actions with a rapid onset of action (0.5 h). 1 In an in vitro whole blood assay in cats, robenacoxib demonstrated selective COX-2 inhibition. 2 The clinical relevance of this data has not been shown. Robenacoxib is an analog of diclofenac.
Absorption
Peak blood concentrations of robenacoxib are attained rapidly after subcutaneous injection in cats. After a dosage of 2 mg/kg, a Tmax of 1 hour, a Cmax of 1464 ng/ml and an AUC(0-inf) of 3128 ng.h/ml is obtained. The injection has a systemic bioavailability of 69%, which is higher than that observed after an equivalent oral dose. A slightly less than dose proportional increase in exposure was observed with an increase in dose (a 2X dose resulted in a 1.4X increase in drug exposure; a 3X increase in dose resulted in a 2.3X increase in drug exposure).
Distribution
Robenacoxib has a relatively small volume of distribution (mean Vss = 190 ml/kg) and is highly bound to plasma proteins (>99%). Robenacoxib persists longer in the inflammatory exudate of a tissue cage model than in blood. The median robenacoxib elimination half-life in exudate was about 27 hours versus 2.5 hours for blood.
Metabolism
Robenacoxib is extensively metabolized by the liver in cats. Apart from one lactam metabolite, the identity of other metabolites is not known.
Elimination
Robenacoxib is rapidly cleared from blood (mean clearance [CL] = 0.44 L/kg/h) with an elimination mean half-life (t1/2) of 1.1 hours after intravenous administration. After subcutaneous administration, the terminal half-life was 1.1 hour (range of estimated values was 0.9 hour to 1.6 hours). Repeated subcutaneous administration at dosages of 2-20 mg/kg produced no change in the blood profile, with neither bioaccumulation of robenacoxib nor enzyme induction. Elimination occurs predominantly through the biliary route (fecal and urinary excretion are 60 and 16.5% respectively). The pharmacokinetics of robenacoxib injection does not differ between male and female.
-
Animal Safety:
3-day Target Animal Safety Study: In a laboratory study, ONSIOR (robenacoxib) injection was administered subcutaneously once daily for 3 days at 5 times the labeled dose (5X = 10 mg/kg). Treatment was associated with vomiting, soft feces/diarrhea, and injection site swelling which disappeared within 72 hours following injection. Histologically, minimal to slight inflammatory cell infiltration in the subcutaneous tissue underlying the injection site was noted in most treated and saline-control cats. Minimal to moderate, locally extensive subcutaneous muscle necrosis and signs of muscle regeneration were seen in 35/37 biopsies of the injection sites in treated cats and in none of the control cat biopsies. Several treated cats had clinically significant elevations in creatine kinase (CK) levels compared to the control cats.
37-Day Interchangeable Use Study: ONSIOR was administered orally (6 mg tablets) and subcutaneously (20 mg/mL solution) to 4-month old healthy cats at 0, 1, 2, and 3 times the labeled doses (1X = 2.4 mg/kg/day orally based on the inherent tablet dose band or 2.0 mg/kg/day subcutaneously). Interchangeable use was evaluated by alternating three 7-day oral tablet/3-day subcutaneous injection cycles followed by one final 7-day oral tablet dosing cycle. All cats survived until study termination. Findings included: elevated creatine kinase levels on Days 13 and 37, soft stools, histologic observation of a minimal oral (tongue) ulceration in a 1X cat, injection site edema for up to 120 hours prior to resolution, and a prolonged QT interval in treated cats as compared to the controls on Day 36. There was a dose dependent and statistically significant increase in the QT interval at all three ONSIOR tablets/ONSIOR injection treatment levels. It is unknown if the increased QT interval suggests an elevated risk of ventricular arrhythmia or torsade de pointes in cats. In addition, a longer PR interval was noted in 1X group females on Day 36 compared to the controls. One 2X cat vomited twice during the last 2 dosing days. Histologically, the injection sites had minimal or mild, subacute/chronic inflammation. Inflammation at the injection sites was observed in both treated and control animals with a greater frequency in the higher dose groups than in the control and 1X groups. Dose-normalized AUC and concentration levels were higher following the oral route than the subcutaneous route. There was no significant accumulation following once daily administration. One 2X-treated cat had a 7-fold increase in buccal mucosal bleeding time (BMBT) during the treatment period compared to the pre-treatment value.
Preliminary 37-Day Interchangeable Use Study: ONSIOR was administered orally
(6 mg tablets) and subcutaneously (20 mg/mL solution) to 4-month old cats at 0, 1, and 5 times the labeled doses (1X = 2.4 mg/kg/day orally based on the inherent tablet dose band or 2.0 mg/kg/day subcutaneously). Interchangeable use was evaluated by alternating three 7-day oral tablet/3-day subcutaneous injection cycles followed by one final 7-day oral tablet dosing cycle. Clinical findings included: scabs and sores at the injection sites of one 1X female and two 5X females, and injection site edema noted more frequently in treated cats. Injection site changes were characterized as minimal to moderate granulomatous inflammation, minimal to moderate fibroplasia/fibrosis, and minimal myofiber regeneration of the panniculus carnosus. In one 1X female, moderate necrosis of a blood vessel was noted within the granulomatous inflammation. Minimal myofiber regeneration was observed in the underlying skeletal muscle in three out of four 5X males.
A red depressed area on the upper lip of one 5X cat correlated histologically with a minimal ulcer. Creatinine was significantly increased in 5X cats compared to the controls. Urine specific gravities remained within normal limits for all 5X cats, and blood urea nitrogen (BUN) values remained within normal limits for all study animals. Histologically, renal changes included bilateral or unilateral minimal to moderate vacuolation and bilateral or unilateral minimal to mild degeneration of proximal tubules were observed in three 5X males. Two 5X males had mineralized foci in the epithelium covering the papilla.
One 5X female had a brief episode of ataxia and lethargy on Day 16. This cat was subsequently noted to be dehydrated and constipated, requiring veterinary intervention with subcutaneous fluid therapy and nutritional supplementation. This cat had the greatest QT increase on ECG evaluation.
-
Effectiveness:
Effectiveness was demonstrated using ONSIOR (robenacoxib) injection in a masked, placebo-controlled, multi-site field study involving client-owned cats. In this study, 349 cats presenting for ovariohysterectomy or castration in conjunction with an onychectomy (forelimbs only) were randomly administered ONSIOR injection or saline. Drug was administered approximately 30 minutes prior to surgery along with pre-anesthetic medications and continued once daily for two additional treatments. Effectiveness was evaluated in 348 cats (173 treated and 175 controls) and field safety was evaluated in 349 cats. A statistically significant difference in the proportion of treatment successes in the ONSIOR injection treatment group (83.5%) compared to the control group (61.9%) was observed. Statistically significant differences for pain elicited on palpation at the spay or castration incision site, paw pain, behavior following social interaction and from a distance, posture score and overall pain at various postsurgical time points were also observed. The results of the field study demonstrate that ONSIOR (robenacoxib) injection when administered for a maximum of 3 days is effective and well-tolerated for the control of postoperative pain associated with onychectomy, ovariohysterectomy, and castration in cats.
- How Supplied:
- Storage Conditions:
-
References:
1Giraudel, J.M., King J.N., Jeunesse, E.C., Lees, P. & Toutain, P.L. (2008). Use of a pharmacokinetic/pharmacodynamic approach in the cat to determine a dosage regimen for the COX-2 selective drug robenacoxib. J Vet Pharmacol Ther 2009;32:18-30.
2Giraudel, J.M., Toutain, P.L., King J.N., & Lees, P. Differential inhibition of cyclooxygenase isoenzymes in the cat by the COX-2 selective drug robenacoxib. J Vet Pharmacol Ther 2009;32:31-40.
NADA # 141-443, Approved by FDA
Elanco, Onsior and the diagonal bar are trademarks owned or licensed by Eli Lilly and Company, it subsidiaries or affiliates.
- Onsior(robenacoxib) injection20 mg/mL injection
- SPL UNCLASSIFIED SECTION
- Caution:
-
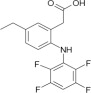
Description:
ONSIOR (robenacoxib) injection is a non-narcotic, non-steroidal anti-inflammatory drug (NSAID) of the coxib class. Each mL of this clear sterile solution for injection contains 20 mg robenacoxib, 350 mg polyethylene glycol 400, 120 mg ethanol, 100 mg poloxamer 188, 1 mg sodium metabisulfite; citric acid and sodium hydroxide are used for pH adjustment. The molecular weight of robenacoxib is 327.28. The empirical formula is C16-H13-F4-NO2. Robenacoxib is [5-Ethyl-2-(2,3,5,6-tetrafluoro-phenylamino)-phenyl]-acetic acid. The structural formula is:
- Indication:
-
Dosage and Administration:
Carefully consider the potential benefits and risks of ONSIOR and other treatment options before deciding to use ONSIOR (robenacoxib) injection. Use the lowest effective dose for the shortest duration consistent with individual response. It is recommended as good aseptic practice to clean stopper surface with isopropyl alcohol before and after each insertion of a needle.
The dose of ONSIOR (robenacoxib) injection is 0.91 mg/lb (2 mg/kg) subcutaneously once daily, for a maximum of 3 days (See Warnings, Precautions and Adverse Reactions).
Dosage Directions: For subcutaneous injection in dogs ≥ 4 months of age; for up to a maximum of 3 days. The first dose should be administered approximately 45 minutes prior to surgery, at the same time as the pre-anesthetic agents are given.
Subsequent doses can be given via subcutaneous injection, or interchanged with the oral tablet in dogs ≥ 5.5 lbs and ≥ 4 months of age, for a maximum of 3 total ONSIOR doses over 3 days, not to exceed one dose per day (see Animal Safety and ONSIOR tablet product insert). If subsequent doses are given by subcutaneous injection, different sites for each injection should be used (See Adverse Reactions).
- Contraindications:
-
Warnings:
Not for use in humans. Keep this and all medications out of reach of children. Consult a physician in case of accidental exposure to humans. For subcutaneous use in dogs and cats only. See reverse side for instructions for use in cats.
Do not administer ONSIOR injection or tablets in conjunction with any other oral or injectable NSAID or corticosteroid.
Do not use for more than 3 days. Serious adverse events have been reported, including hepatopathy, with the long term use (28 day study) of ONSIOR tablets in dogs (See Adverse Reactions). Safety not demonstrated for longer than 3 days.
All dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory tests should be conducted to establish hematological and serum biochemical baseline data prior to administration of an NSAID. Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions and Animal Safety).
-
Precautions:
Monitor dogs post-injection for reactions. Injection site reactions and anaphylactic reactions have been associated with the use of ONSIOR injection (See Adverse Reactions).
Stop administration of ONSIOR if the dog experiences inappetence, vomiting or lethargy.
The safe use of ONSIOR has not been evaluated in dogs younger than 4 months of age, dogs used for breeding, or in pregnant or lactating dogs. Safety has not been demonstrated for intravenous or intramuscular administration of ONSIOR injection.
As a class, cyclo-oxygenase inhibitory NSAIDS may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Anesthetic drugs may affect renal perfusion; approach concomitant use of anesthetics and NSAIDS cautiously. Appropriate monitoring procedures (including ECG, blood pressure, and temperature regulation) should be employed during all surgical procedures. The use of parenteral fluids during surgery is recommended to decrease potential renal complications when using NSAIDS perioperatively.
If additional pain medication is needed after a daily dose of ONSIOR, a non-NSAID/ non- corticosteroid class of analgesic may be necessary. Concurrent administration of potentially nephrotoxic drugs should be carefully approached and monitored. NSAIDs may inhibit prostaglandins which maintain normal homeostatic function. Such antiprostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. NSAIDs possess the potential to produce gastrointestinal ulcerations and/or gastrointestinal perforations. Do not use ONSIOR concomitantly with other anti-inflammatory drugs, such as NSAIDs or corticosteroids. Consider appropriate washout times when switching from one NSAID to another or when switching from corticosteroid use to NSAID use. ONSIOR injection and ONSIOR tablets are safe to use interchangeably when given once a day for a maximum of 3 days in dogs ≥ 4 months of age and ≥ 5.5 lbs.
The use of concomitantly protein-bound drugs with ONSIOR has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant, and behavioral medications. The influence of concomitant drugs that may inhibit metabolism of ONSIOR has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy. Concurrent medications used during the field study with ONSIOR included parasiticides, anesthetics, pre-anesthetic medications, and antibiotics.
It is unknown whether dogs with a history of hypersensitivity to ß lactam drugs will exhibit hypersensitivity to ONSIOR. Robenacoxib is poorly soluble in water and in acid solutions readily degrades to form γ-lactam. In dogs, lactam is a minor metabolite of robenacoxib. Additionally, lactam is a degradation product that increases over the shelf life of the solution. Neurologic signs have been associated with the use of ß lactam drugs; it is unknown if the lactam produced by robenacoxib may cause similar neurologic signs.
ONSIOR (robenacoxib) injection contains sodium metabisulfite. ONSIOR (robenacoxib) injection should not be used in patients with sulfite hypersensitivity. A sulfite may cause allergic-type reactions including anaphylaxis.
-
Adverse Reactions:
In a controlled field study (See Effectiveness), a total of 317 male and female dogs representing various breeds were included in the field safety analysis. ONSIOR-treated dogs ranged in age from 6 months to 15 years and weighed between 2.5 and 53.8 kg. The following table shows the number of dogs exhibiting each observation.
Table 1: Adverse reactions reported in the soft tissue surgery field study. * Dogs may have experienced more than one type or occurrence of an event during the study.
**Most often occurred as a single event.
Adverse Reaction* ONSIOR
(robenacoxib) injection
N = 159Placebo (0.9% NaCl)
N = 158Pain on injection** 18 8 Diarrhea 15 8 Vomiting 10 6 Bradycardia 6 1 Decreased appetite 5 2 Hypotension 2 0 Facial edema, hypersensitivity 1 0 Increased incisional bleeding 1 0 Pain on injection, diarrhea and vomiting were the most commonly reported adverse reactions.
Occurrences of hepatopathy, ataxia, skin lesions/urticaria, and anaphylaxis have been associated with the use of ONSIOR. In a month-long pilot study, 3 dogs that received ONSIOR developed hepatic toxicity. Two of these dogs were euthanized and a third dog recovered after prolonged hospitalization and supportive therapy. In foreign market experience, elevated liver enzymes, hepatic necrosis and death have been associated with the long term use of robenacoxib in dogs. Occurrences of liver failure, hepatitis, and cholangiohepatitis have been reported. In two other field studies, bradycardia, 2nd degree heart block in four dogs, and ventricular arrhythmia in one dog were noted in anesthetized dogs treated with ONSIOR injection.
Injection site reactions (edema, necrosis, and abscesses), and anaphylactic reactions (panting, drooling, shock, pallor, dyspnea, tachypnea, ataxia) have been associated with the use of ONSIOR injection.
For technical assistance or to report suspected adverse drug events, contact Elanco US Inc. at 1-888-545-5973. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/AnimalVeterinary/SafetyHealth
-
Information for Dog Owners:
ONSIOR, like other drugs of its class, is not free from adverse reactions. Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include vomiting, diarrhea, decreased appetite, dark or tarry stools, increased water consumption, increased urination, anemia, yellowing of gums, skin or whites of the eye due to jaundice, lethargy, incoordination, seizure, or behavioral changes. Serious adverse reactions associated with this drug class can occur without warning and in some cases result in death (see Warnings and Adverse Reactions). Owners should be advised to discontinue ONSIOR therapy and contact their veterinarian immediately if signs of intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated.
-
Clinical Pharmacology:
Robenacoxib is a non-steroidal anti-inflammatory drug (NSAID) of the coxib class. Repeated subcutaneous administration is not associated with a bioaccumulation of robenacoxib. Although the bioaccumulation of robenacoxib metabolites has not been tested, radiolabeled studies suggest that the various unidentified metabolites are associated with a markedly longer elimination half-life (approximately 22 hours) as compared to that of the parent molecule. The activity of these metabolites has not been evaluated. The pharmacokinetics of robenacoxib injection does not differ between male and female dogs, and is linear over the range of 0.25-4 mg/kg in dogs.
-
Absorption
Peak blood concentrations of robenacoxib are attained rapidly after subcutaneous injection in dogs. After a dosage of 2 mg/kg, peak concentrations are generally achieved within 1-2 hours post-dose. After subcutaneous administration of 1 mg/kg to dogs, systemic bioavailability ranged from 67% to 100% (average = 89%).
-
Distribution
Robenacoxib has a relatively small volume of distribution (Vss of 174 to 336 mL/kg, average = 240 mL/kg), which is consistent with a drug that is extensively bound to plasma proteins. Based upon in vitro canine plasma samples, robenacoxib is approximately 98% bound to plasma proteins when spiked at concentrations ranging from 200 – 2000 ng/mL. The corresponding canine in vitro whole blood-plasma ratio in artificially spiked blood was 0.44.
-
Biotransformation
Robenacoxib is extensively metabolized by the liver in dogs. Apart from one lactam metabolite, the identity of other metabolites is not known in dogs. Robenacoxib is excreted predominately via the biliary route in dogs (approximately 65 %); therefore, the majority of the absorbed dose (parent drug and metabolites) is eliminated in the feces. The remainder of the administered dose is eliminated in urine via the kidneys.
-
Elimination
After intravenous administration to normal healthy dogs, robenacoxib is rapidly cleared from blood (CL of 0.61 to 1.13 L/kg/h, average = 0.81 L/kg/hr) with an elimination half-life ranging from 0.41 to 1.07 hrs. After subcutaneous administration to normal healthy dogs, the terminal half-life from blood was within 1 – 4 hrs.
-
Animal Safety:
88-Day Interchangeable Use Study: In an 88-day laboratory interchangeable use study, 4-month old healthy mongrel dogs (4 sex/dose) were administered three 20 day cycles (separated by a 14-day washout) of alternating regimens of ONSIOR tablets and ONSIOR injection. Each cycle included a schedule of 7 days once daily oral tablet administration (0, 2, 4, or 6 mg/kg/day; Groups 1, 2, 3, and 4, respectively), 3 days of once daily oral administration (0, 4, 8, or 12 mg/kg/day; Groups 1, 2, 3, and 4, respectively), 3 days of once daily subcutaneous injection (0, 4, 8, or 12 mg/kg/day; Groups 1, 2, 3, and 4, respectively), and then 7 days once daily oral administration (0, 2, 4, or 6 mg/kg/day; Groups 1, 2, 3, and 4, respectively). The negative control group (Group 1) received empty gelatin capsules or saline injection.
All mongrels were in good health through study termination. Injection site reactions, including skin thickening, ulceration, or granulation, occurred in dogs in all groups in a dose-dependent manner, including one control dog. Histologically, there was minimal to severe subcutaneous necrosis, degeneration, and/or fibrosis with occasional myonecrosis of the underlying panniculus muscle. On gross pathology, one dog in Group 2 had discoloration throughout the entire duodenal, jejunal, and ileal mucosa, as well as multiple mucosal discolorations in the stomach, with no corresponding histopathology findings, except for a jejunal ulcer with minimal inflammation. Another dog in Group 2 had stomach, duodenal and jejunal mucosal discoloration with no corresponding histopathology findings. One Group 3 dog had multiple mucosal discoloration in the stomach and duodenum with no histopathology findings; and microscopic minimal cecal hemorrhage with microscopic cecal inflammation. This dog also vomited on 2 days. Another dog in Group 3 had discoloration grossly along the entire duodenal and jejunal mucosa with no correlating histopathology findings; a single mucosal discoloration in the stomach with no histopathology findings, and slight duodenal congestion microscopically. This dog vomited on 3 study days. Microscopic cecal inflammation was noted in one Group 4 dog. There were no gastrointestinal findings noted in the control group. Treated male dogs exhibited an increased number and severity of lymphocyte depletion within the thymus compared to the controls.
3-Day Target Animal Safety Study: Injection site tolerance was evaluated in 5-7.5 year old, healthy Beagles. Subcutaneous administration of ONSIOR 20 mg/mL injection at 0 (saline), 2 mg/kg (1X), or 10 mg/kg (5X) once a day for 3 days (4/sex/dose) resulted in transient, fluid-filled, swellings which resolved within 72 hours. Histologically, ONSIOR-injected sites had minimal to slight subcutaneous inflammatory cell infiltration in the subcutis and regenerating, minimal to mild acute myonecrosis at 2 mg/kg and 10 mg/kg.
16-Day Target Animal Safety Study: After ONSIOR administration intravenously (IV) at 0 (saline), 2 mg/kg (1X) and 4 mg/kg (2X), and subcutaneously (SC) at 2 mg/kg (at least 72 hours apart) to healthy Beagles (4/sex; 1.5 - 3 years old), there were no effects on arterial blood pressure, heart rate, or cardiac conduction times during 8-hours of postdosing for cardiovascular telemetric measurements. No arrhythmia attributable to the test article was observed. Two dogs vomited after IV administrations at 4 mg/kg.
-
Effectiveness:
Effectiveness was demonstrated using ONSIOR (robenacoxib) injection in a masked, placebo-controlled, multi-site field study in which 317 client-owned dogs presenting for soft tissue surgery were randomized to receive ONSIOR injection or placebo. Drug was administered approximately 45 minutes prior to surgery, along with pre-anesthetic medications, and continued once daily for two additional treatments. Effectiveness was evaluated in 303 dogs and field safety was evaluated in 317 dogs. A statistically significant difference (p-value = 0.0055) was observed in the proportion of treatment successes in the ONSIOR injection treatment group (108/151; 73.7%) compared to the placebo group (85/152; 58.1%). Forty three of 151 dogs in the ONSIOR injection group and 67 of 152 dogs in the placebo group were treatment failures. Statistically significant differences in reductions in Response to Touch scores (p = 0.0013) and Posture/Activity (p = 0.0466) were observed in the ONSIOR injection group relative to the placebo group. The results of the field study demonstrate that subcutaneous administration of ONSIOR (robenacoxib) injection, when administered for a maximum of 3 days, is effective and well-tolerated for the control of postoperative pain associated with soft tissue surgery in dogs.
- How Supplied:
-
Storage Conditions:
Store under refrigerated conditions 2° - 8°C (36° - 46°F). Use within 12 weeks of first puncture.
Manufactured for: Elanco US Inc. Greenfield, IN 46140
NADA # 141-443, Approved by FDA
Elanco, Onsior and the diagonal bar are trademarks owned or licensed by Eli Lilly and Company, it subsidiaries or affiliates.
PA100455AMX
Elanco
-
Principal Display Panel – Carton Label
onsior™
(robenacoxib)
injection
20 mg/mL
For subcutaneous use
in Dogs and Cats OnlyA Coxib Class Non-Steroidal Anti-inflammatory
Net contents:
1 multi-dose vial
containing 20 mL
CAUTION: Federal (USA) law restricts
this drug to use by or on the order
of a licensed veterinarian.NADA 141-443, Approved by FDA
Elanco™
20 mg/mL
- Principal Display Panel – 20 mg Vial Label
-
INGREDIENTS AND APPEARANCE
ONSIOR
robenacoxib injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 58198-4886 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength robenacoxib (UNII: Z588009C7C) (robenacoxib - UNII:Z588009C7C) robenacoxib 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength polyethylene glycol 400 (UNII: B697894SGQ) alcohol (UNII: 3K9958V90M) poloxamer 188 (UNII: LQA7B6G8JG) SODIUM DITHIONATE (UNII: RPF7Z41GAW) citric acid monohydrate (UNII: 2968PHW8QP) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-4886-1 1 in 1 CARTON 1 20 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141443 08/05/2015 Labeler - Elanco US Inc, (966985624)
Trademark Results [Onsior]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ONSIOR 87234459 5214181 Live/Registered |
ELANCO US INC. 2016-11-11 |
 ONSIOR 79024304 3223362 Dead/Cancelled |
Novartis Tiergesundheit AG 2006-04-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.