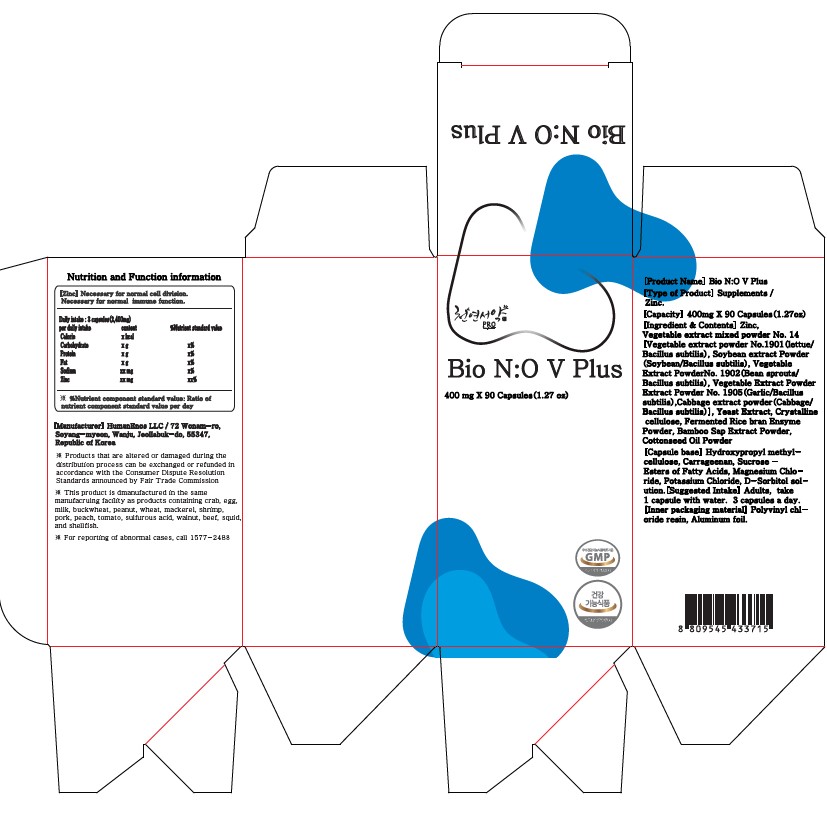
Bio NO V Puls (Helps improve matabolic disorder, Bean sprout powder)
Bio NO V Puls (Helps improve matabolic disorder, Bean sprout powder) by
Drug Labeling and Warnings
Bio NO V Puls (Helps improve matabolic disorder, Bean sprout powder) by is a Otc medication manufactured, distributed, or labeled by HumanEnos LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BIO NO V PULS (HELPS IMPROVE MATABOLIC DISORDER, BEAN SPROUT POWDER)- nitric oxide, zinc, glutathione capsule
HumanEnos LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Bio NO V Puls (Helps improve matabolic disorder, Bean sprout powder)
| BIO NO V PULS (HELPS IMPROVE MATABOLIC DISORDER, BEAN SPROUT POWDER)
nitric oxide, zinc, glutathione capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - HumanEnos LLC (695801540) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HumanEnos LLC | 695801540 | manufacture(83202-9725) | |
Revised: 3/2023
Document Id: f619f146-fb5f-133c-e053-2995a90a2658
Set id: f50241c9-aa6c-7735-e053-2a95a90a317d
Version: 2
Effective Time: 20230304
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.