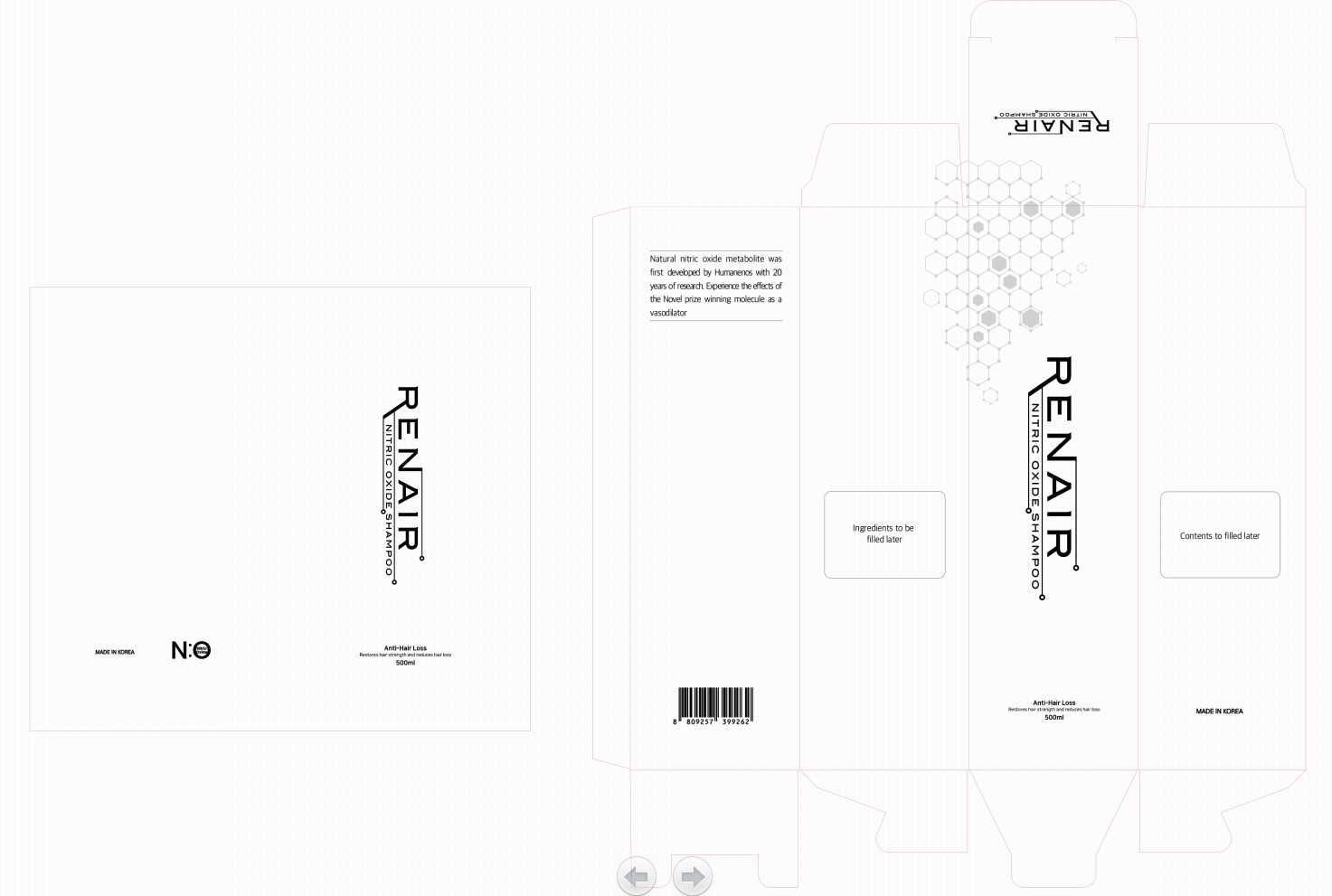
Renair Anti-hairloss Shampoo (Prevents hairloss, Fermented lettuce, garlic, bean, cabbage, cricket Greentea leaf, Mulberry branch, leaf, and roots extract)
Renair (Prevents hairloss, fermented lettuce, garlic, bean, cabbage, cricket greentea leaf,mulberry branch, leaf, and roots extract) by
Drug Labeling and Warnings
Renair (Prevents hairloss, fermented lettuce, garlic, bean, cabbage, cricket greentea leaf,mulberry branch, leaf, and roots extract) by is a Otc medication manufactured, distributed, or labeled by HumanEnos LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RENAIR (PREVENTS HAIRLOSS, FERMENTED LETTUCE, GARLIC, BEAN, CABBAGE, CRICKET GREENTEA LEAF,MULBERRY BRANCH, LEAF, AND ROOTS EXTRACT)- nitric oxide, dexpanthenol, niacinamide, salicylic acid, zinc liquid
HumanEnos LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Renair Anti-hairloss Shampoo (Prevents hairloss, Fermented lettuce, garlic, bean, cabbage, cricket Greentea leaf, Mulberry branch, leaf, and roots extract)
| RENAIR (PREVENTS HAIRLOSS, FERMENTED LETTUCE, GARLIC, BEAN, CABBAGE, CRICKET GREENTEA LEAF,MULBERRY BRANCH, LEAF, AND ROOTS EXTRACT)
nitric oxide, dexpanthenol, niacinamide, salicylic acid, zinc liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - HumanEnos LLC (695801540) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HumanEnos LLC | 695801540 | manufacture(83202-7412) | |
Revised: 3/2023
Document Id: f619fda8-c40b-1ce8-e053-2995a90ae1a8
Set id: f5161adb-c51e-7cb6-e053-2995a90a7320
Version: 2
Effective Time: 20230304
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.