THIANIL- thiafentanil oxalate injection, solution
Thianil by
Drug Labeling and Warnings
Thianil by is a Animal medication manufactured, distributed, or labeled by Clovis-Davis Pharmaceuticals LLC, Wildlife Laboratories Inc, Wildlife Pharmaceuticals Pty Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
PATIENT PACKAGE INSERT
Thianil
(thiafentanil oxalate)
Injectable solution
10 mg/mLCII
For intramuscular injection in captive non-food-producing minor
species hoof stock only.
CAUTION: Federal (USA) law restricts this drug to use by or on the
order of a licensed veterinarian.
NOT APPROVED BY FDA Legally marked as an FDA Indexed
Product under MIF 900 000. Extra label use prohibited.
Note: In order to be legally marketed an animal drug product
intended for a minor species must be Approved, Conditionally
Approved, or Indexed by the FDA. THIS PRODUCT IS INDEXED.
Do not use this product without adequate amounts of reversal
agent available.It is a violation of Federal Law to use this product in a manner other
than as directed in the labelling. The term minor species means
animals other than humans that are not major species. Major
species means cattle, horses, swine, chickens, turkeys, dogs,
and cats. As used on this label, a food-producing minor species
is considered to be a minor species of which some members
are bred, cultured, farmed, ranched, hunted, caught, trapped or
otherwise harvested for the purpose of having the animals or edible
products of the animals commercially distributed for consumption
by humans or food-producing animals in the United States.
- DESCRIPTION:
- PHARMACOLOGY:
-
INDICATION:
For immobilization of captive minor species hoof
stock, excluding any member of a food-producing minor species
such as deer, elk or bison and any minor species animal that may
become eligible for consumption by humans or food-producing
animals.
Use only when there is a reasonable certainty that the treated
animal will not be consumed by humans or food-producing
animals. -
DOSAGE AND ADMINISTRATION:
Total doses used in captive minor species hoof stock to date have ranged from 1 mg (Impala)
to 15 mg (African elephant). Immobilization is usually achieved in
2 to 10 minutes following administration. The most effective dose
rate will vary due to the conditions of use.The lower end of the dose range is suggested for those animals of quiet
temperament, under confinement, that have not been hotly pursued
prior to administration of the drug, or for animals in poor physical condition.
The upper end of the dose range is suggested for animals of
excitable temperament following extensive pursuit or in instances
where an extremely short chase time is desirable. The upper end of
the dose range may also be appropriate for animals being pursued
by vehicle or aircraft when an extremely quick immobilization time
is desired or when individuals are known to be highly excitable.
In all instances, all factors including nutritional, reproductive, and
health status of an animals as well as environmental conditions
(temperature, cover, and terrain) must be evaluated by the used
and the best professional judgement used.DOSE CHART
The following doses are representative of doses used in field trials.
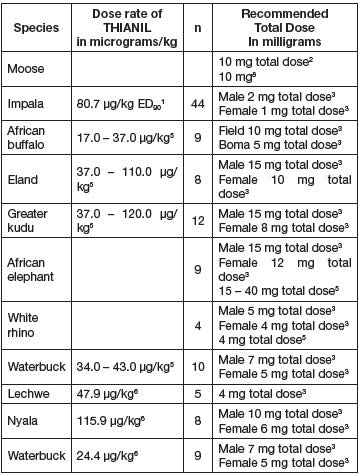
1 Janssen, DL, GE Swan, JP Raath, SW McJames, JL Allen,
V de Vos, KE Williams, JM Anderson, and TH Stanley. 1993.
Immobilization and physiologic effects of narcotic A-3080 in
impala (Aepyceros melampus). J. Zoo Wildl. Med., 24: 11-18.
2 Kreeger, TJ and JM Arnemo. 2007. Handbook of Wildlife Chemical
Immobilization, 3rd ed. For North and South American orders:
tkreeger@starband.net or Amazon.com.
3 Lance, WR. 2008 Attachemtn C; Recommended Dosages
for Adult Animals South Africa and North America. Wildlife
Pharmaceuticals, Inc, fort Collins, CO 80524. P. 9-10.
4 McJames, SW, IL Smith, TH Stanley, and G Painter. 1993. Elk
immobilization with potent opioids: A3080 vs. carfentanil. Proc.
Conf. Amer. Assoc. Zoo Vet., Saint Louis, Missouri. pp. 418-419.
5 Raath, JP. 2005. Clinical Expert Report First Study. pp. 1-11.
6 Raath, JP. 2005 Clinical Expert Report Second Study. pp. 11-22.
7 Stanley, TH, SW McJames, J Kimball, JD Port, and NL Pace. 1988.
Immobilization of elk with A-3080. J. Wildl Mgmt. pp. 577-581.
8 Stanley, TH, SW McJames, and J Kimball. 1989. Chemical
immobilization for the capture and transportation of big game.
Proc. Conf. Amer. Assoc. Zoo Vet. Greensboro, North Carolina.
pp. 13-14.
Inject dose deep into a large muscle mass of the neck, shoulder, back,
or hindquarter. Intra-thoracic, intra-abdominal, or subcutaneous
injection is to be avoided. To ensure proper dosage for animals
weighing less than 50 kg, remove the calculated dose of THIANIL
from the vial with a tuberculin syringe. Dilute appropriate volume with
sterile water for injection prior to administration. Operator should use
safe technique by working in pairs, wearing disposable latex gloves,
and wearing eye protection. Used syringes should be secured and
disposed of in an appropriate biohazard container. -
ANTIDOTE:
TREXONILTM (naltrexone hydrochloride) is the recommended
antidote and rapidly reverses the effects of THIANIL.
Administer 10 mg TREXONILTM for each milligram of THIANIL.
The total calculated dose of TREXONILTM should be administered
intramuscular unless there is a medical or anesthetic emergency
requiring immediate reversal, then ¼ of the calculated dose should
be administered intravenously and ¾ of the calculated dose should
be administered intramuscularly. The entire dose can be safely
delivered intravenously but the operator should be prepared for
occasional extrapyramidal activity and signs, and/or very rapid
return to consciousness and mobility. Reversal of effects of THIANIL
are usually observed in 2 to 10 minutes, with differences resulting
from whether reversal is performed by intramuscular injection,
split between intravenous injection and intramuscular injection, or
completely delivered by intravenous injection. -
CONTRAINDICATIONS:
THIANIL is not for use in minor species
hoof stock in the family Equidae. Do not use thiafentanil oxalate
in animals that display clinical signs of disease unless its use is
imperative to establish a diagnosis and/or administer therapeutic
agents. The effects of thiafentanil oxalate on reproductive
performance, pregnancy, and lactation have not been determined.
An opioid antagonist should always be drawn up, labelled, and
readily accessible prior to drawing up thiafentanil oxalate. -
WARNINGS:
As with other opioids, all species immobilized with
THIANIL may show signs of excitement, tachycardia or bradycardia,
tachypnea or bradypnea, hypertension or hypotension, depressed
respiration, cyanosis, poikilothermia, and reaction to sudden noise.
Personnel should be advised of these potential opioid effects and
trained to respond appropriately. -
PRECAUTIONS:
THIAFENTANIL OXALATE SHOULD NEVER BE
USED UNLESS AN ADEQUATE AMOUNT OF THE REVERSAL
AGENT, TREXONILTM (naltrexone hydrochloride), IS IMMEDIATELY
AVAILABLE. Veterinarians using THIANIL should be familiar with
clinical procedures such as measurement of pulse and respiration,
oxygen saturation, prevention of aspiration, relief of bloat, obstetrics,
control of shock and hemorrhage, recognition for hyperventilation,
heat exhaustion, capture myopathy, and the immobilization of
fractures, etc. In cases of severe excitement during induction or
delayed recovery, continued observation is necessary to correct any
of the above situations and to insure the animal does not injure itself. - STORAGE:
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
-
CARTON
VETERINARY MEDICINE
Thianil
(thiafentanil oxalate)
injectable solution10mg/mL
Immobilization Agent
CII
Wildlife PharmaceuticalsTM
Distributed by:
Clovis-Davis Pharmaceuticals, LLC
2518 Burnsed Blvd, Ste 610 The Villages, FL 32163
833-878-0060CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
For intramuscular injection in captive non-food producing minor species hoof stock only.
NOT APPROVED BY FDA - Legally marketed as an FDA Indexed Product under MIF 900 000.
Extra label use is prohibited.Net Contents: 10mL
NDC NO. 86204-729-10
INDICATION: For immobilization of captive minor species hoof stock, excluding any member of a food-producing minor species such as deer, elk, or bison and any minor species animal that may become eligible for consumption by humans or food-producing animals.
DOSE & DIRECTIONS: Before using, read the package insert for complete product information. Do not use without adequate amounts of reversal agent available.
WARNING: Not for use in humans.
KEEP OUT OF THE REACH OF CHILDREN.
Storage: Protect from sunlight.
Store at controlled room temperature
15 °C - 30 °C (59 °F - 86 °F).
-
PRINCIPAL DISPLAY PANEL
VETERINARY MEDICINE
Thianil
(thiafentanil oxalate)
injectable solutionStore at controlled room temperature
15 °C - 30 °C (59 °F - 86 °F)Wildlife PharmaceuticalsTM
Distributed by:
Clovis-Davis Pharmaceuticals, LLC
2518 Burnsed Blvd, Ste 610 The Villages, FL 32163
833-878-006010mg/mL
Immobilization Agent
CII
For intramuscular injection in captive non-food producing minor species hoof stock only.
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Net Contents: 10mL
For use in captive non-food -producing minor species hoof stock only.
Do not use without adequate amounts of reversal agent available.
Before using, read the package insert for complete product information.
NOT APPROVED BY FDA - Legally marketed as an FDA Indexed Product under MIF 900 000.
Extra label use is prohibited.NDC NO. 86204-729-10
-
INGREDIENTS AND APPEARANCE
THIANIL
thiafentanil oxalate injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 86204-729 Route of Administration INTRAMUSCULAR DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THIAFENTANIL OXALATE (UNII: 5Z3JX123QJ) (THIAFENTANIL - UNII:HS2D307FGT) THIAFENTANIL OXALATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Methylparaben (UNII: A2I8C7HI9T) 1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 86204-729-10 10 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date legally marketed unapproved new animal drugs for minor species MIF900000 09/01/2024 Labeler - Clovis-Davis Pharmaceuticals LLC (119008775) Registrant - Wildlife Laboratories Inc (055417281) Establishment Name Address ID/FEI Business Operations Wildlife Pharmaceuticals Pty Ltd 639405794 manufacture, api manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.