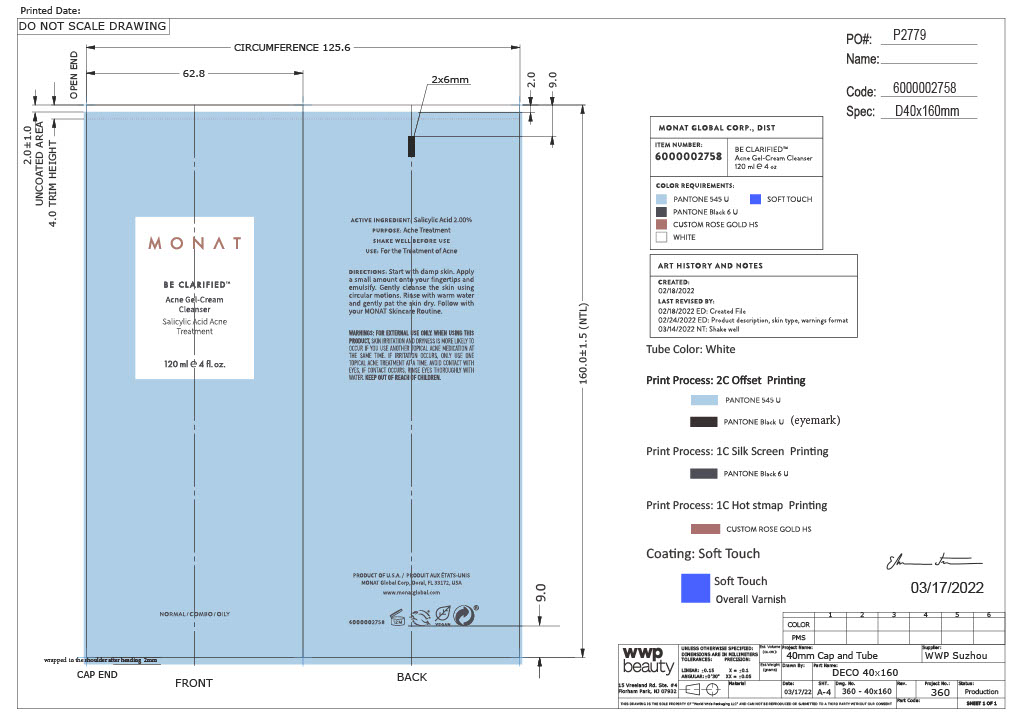
MONAT Be Clarified Acne Gel-Cream Cleanser
ACNE CLEANSER by
Drug Labeling and Warnings
ACNE CLEANSER by is a Otc medication manufactured, distributed, or labeled by MONAT GLOBAL CORP, Oxygen Development. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ACNE CLEANSER- salycilic acid gel
MONAT GLOBAL CORP
----------
MONAT Be Clarified Acne Gel-Cream Cleanser
For external use only
Directions
Directions
- Shake well before use
- Start with damp skin
- Apply small amount onto your fingertips and emulsify
- Gently cleanse the skin using circular motions.
- Rinse with warm water and gently pat the skin dry
- Follow with your MONAT skincare Routine
Inactive Ingredients
Water, Sodium Cocoyl Glutamate, Propanediol, Sorbeth-230 Tetraoleate, Cocamidopropyl Betaine, Sodium C14-16 Olefin Sulfonate, Sodium Cocoyl Isethionate, PEG-120 Methyl Glucose Dioleate, Niacinamide, Cocamide MIPA, Glycerin, Coconut Acid, Sodium Isethionate, Sodium PCA, Maclura Cochinchinensis Leaf Prenylflavonoids, Glycol Distearate, Laureth-4, Sodium Chloride, Sodium Benzoate, Potassium Sorbate, Polyquaternium-10, Decyl Glucoside, Sorbitan Laurate, Isopropyl Alcohol.
| ACNE CLEANSER
salycilic acid gel |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MONAT GLOBAL CORP (027036949) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Oxygen Development | 137098492 | manufacture(78518-011) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 SECONDARY-OUTER PACKAGE
SECONDARY-OUTER PACKAGE