TERCONAZOLE VAGINAL CREAM 0.8%- terconazole cream
Terconazole by
Drug Labeling and Warnings
Terconazole by is a Prescription medication manufactured, distributed, or labeled by E. Fougera & Co. a division of Fougera Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TERCONAZOLE VAGINAL CREAM safely and effectively. See full prescribing information for TERCONAZOLE VAGINAL CREAM.
TERCONAZOLE vaginal cream,
Initial U.S. Approval: 1987INDICATIONS AND USAGE
Terconazole Vaginal Cream is an azole antifungal indicated for the treatment of vulvovaginal candidiasis in adult females. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Vaginal cream containing terconazole 0.8%: supplied in a 20 gram tube with a measured dose applicator. Each applicator delivers 40 mg of terconazole. (3)
CONTRAINDICATIONS
Known hypersensitivity to terconazole or any other component of the cream (4)
WARNINGS AND PRECAUTIONS
Risk of Skin Irritation and Flu-Like Symptoms: Discontinue Terconazole Vaginal Cream and do not retreat if skin irritation, fever, chills or flu-like symptoms are reported during use. (5)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 2%) were headache, dysmenorrhea, genital burning and itching, and abdominal pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Fougera at 1-800-645-9833 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Skin Irritation and Flu-Like Symptoms
5.2 Hypersensitivity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse reactions that appear to be related to drug use and estimating their relative rates.
During a controlled clinical trial conducted in the United States, patients with vulvovaginal candidiasis were treated with Terconazole Vaginal Cream, 0.8% for 3 days (n=231) or Terazol®3 for 3 days (n=229) [see Clinical Studies (14)]. Table 1 lists the adverse reactions occurring in ≥2% of patients receiving Terconazole Vaginal Cream in the trial. The therapy-related discontinuation rate was 2.0% for Terconazole Vaginal Cream. The adverse reaction most frequently causing discontinuation of Terconazole Vaginal Cream therapy was vulvovaginal itching (0.7%).
Table 1: Adverse Reactions Occurring in ≥2% of Patients Receiving Terconazole Vaginal Cream in a Randomized, Double-Blind Active Controlled Trial
Terconazole Vaginal Cream, 0.8%
n=231 (%)
Terazol®3
n=229 (%)
Headache
49 (21)
37(16)
Dysmenorrhea
14 (6)
5 (2)
Genital Burning and Itching
12 (5)
15-21 (6-9)
Abdominal Pain
8 (3.4)
2 (1)
Other adverse reactions reported in less than 2% of patients receiving Terconazole Vaginal Cream was fever (1%).
Photosensitivity reactions were observed in some normal volunteers following repeated dermal application of terconazole 2.0% (not an approved strength) and 0.8% creams under conditions of filtered artificial ultraviolet light. Photosensitivity reactions were not observed in U.S. and foreign clinical trials in patients who were treated with other terconazole formulations (i.e., terconazole suppositories or vaginal cream, 0.8%).
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Terconazole Vaginal Cream use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Available data from observational studies with terconazole use in pregnancy are insufficient to identify a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
In animal reproduction studies with oral terconazole, no adverse development effects were observed at clinically relevant systemic exposures (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
There was no evidence of malformations when terconazole was administered orally in rats, or rabbits.
There was a delay in fetal ossification at an oral dose of 10 mg/kg/day in rats. Higher doses resulted in decreased litter size, decreased number of viable young and reduced fetal weight in rats. There was also a delay in ossification and an increase incidence of skeletal variants.
The dose of 10 mg/kg/day resulted in a mean peak plasma level of terconazole in pregnant rats of 0.176 mcg/mL which exceeds by 30 times the mean peak plasma level (0.006 mcg/mL) seen in normal subjects after intravaginal administration of terconazole vaginal cream 0.8%. This safety assessment does not account for possible exposure of the fetus through direct transfer to terconazole from the irritated vagina by diffusion across amniotic membranes.
8.2 Lactation
Risk Summary
There are no data on the presence of terconazole in human milk, the effect on the breast-fed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Terconazole Vaginal Cream and any potential adverse effects on the breast-fed infant from Terconazole Vaginal Cream or from the underlying maternal condition.
- 10 OVERDOSAGE
-
11 DESCRIPTION
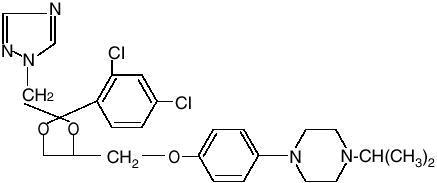
Terconazole Vaginal Cream, 0.8% is a white to off-white, water washable cream for intravaginal administration containing 0.8% of the antifungal agent terconazole, cis-1-[p[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4- yl]methoxy]phenyl]-4-isopropylpiperazine, compounded in a cream base consisting of butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water. The structural formula of terconazole is as follows:
Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Following intravaginal administration of Terconazole Vaginal Cream, 0.8% in adult females, absorption ranged from 5-8% in three hysterectomized subjects and 12-16% in two non-hysterectomized subjects with tubal ligations.
Following daily intravaginal administration of 0.8% terconazole 40 mg (0.8% cream x 5 g) for seven days to healthy female subjects, plasma concentrations were low and gradually rose to a daily peak (mean of 5.9 ng/mL or 0.006 mcg/mL) at 6.6 hours. Results from similar studies in female patients with vulvovaginal candidiasis indicate that the slow rate of absorption, the lack of accumulation, and the mean peak plasma concentration of terconazole was not different from that observed in healthy females. The absorption characteristics of terconazole vaginal cream, 0.8% in pregnant or non-pregnant patients with vulvovaginal candidiasis were also similar to those found in healthy subjects.
Following oral (30 mg) administration of 14C-labelled terconazole, the mean half-life of elimination from the blood for the parent terconazole was 6.9 hours (range 4.0-11.3). Terconazole is extensively metabolized; the plasma AUC for terconazole compared to the AUC for total radioactivity was 0.6%. Total radioactivity was eliminated from the blood with a mean elimination half-life of 52.2 hours (range 44-60). Excretion of radioactivity was both by renal (32-56%) and fecal (47- 52%) routes.
In vitro, terconazole is highly protein bound (94.9%) and the degree of binding is independent of drug concentration.
12.4 Microbiology
Mechanism of Action
Terconazole inhibits the enzyme cytochrome P450 14α-demethylase which leads to inhibition of ergosterol synthesis, an essential component of the fungal cell membrane
Activity in vitro
Terconazole exhibits fungicidal activity in vitro against Candida albicans.
Drug Resistance
Studies showed no development of resistance to terconazole during successive passages of C. albicans. However, the clinical significance of such an effect is not known.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Studies to determine the carcinogenic potential of terconazole have not been performed.
Mutagenesis
Terconazole was not mutagenic when tested in vitro for induction of microbial point mutations (Ames test), or for inducing cellular transformation, or in vivo for chromosome breaks (micronucleus test) or dominant lethal mutations in mouse germ cells.
Impairment of Fertility
No impairment of fertility occurred when female rats were administered terconazole orally up to 40 mg/kg/day (about 10 times the recommended intravaginal human dose based on body surface area comparisons) for a three-month period.
-
14 CLINICAL STUDIES
The efficacy of Terconazole Vaginal Cream, 0.8% in the treatment of vulvovaginal candidiasis in adult women was evaluated in a multicenter, randomized, double-blind, controlled non-inferiority trial comparing Terconazole Vaginal Cream, 0.8% for 3 days (n=231) to another preparation of terconazole vaginal cream 0.8% active comparator for 3 days (n=229). The modified intent-to-treat population (randomized patients who received treatment and had a positive baseline culture for Candida) consisted of 140 Terconazole Vaginal Cream, 0.8% patients and 153 active comparator patients. Therapeutic cure defined as clinical and mycological cure was assessed at Visit 3 (Day 21-30). Table 2 shows the therapeutic, clinical and mycological cure rates in this trial. The therapeutic cure rate at Visit 3 was 67.1% for the terconazole group and 52.3% for the active comparator group (95% confidence interval about the 14.8% difference in therapeutic cure rate: 3.0% to 26.6%).
Table 2: Efficacy of Terconazole Vaginal Cream 0.8% for the Treatment of Vulvovaginal Candidiasis in a Randomized, Double-Blind Active Controlled Study*
Outcome
Terconazole Vaginal Cream 0.8%
n=140 (%)
Active Control
n=153
Treatment Difference (%) [95% Confidence Interval]
% Cure
% Cure
Therapeutic Cure
67.1
52.3
14.8 [3.0, 26.6]
Clinical Cure
79.3
66.0
13.3 [2.5, 24.0]
Mycological Cure
70.7
56.9
13.8 [2.2, 25.4]
* Modified intent-to-treat population
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Terconazole Vaginal Cream 0.8% is supplied in a 20-gram tube with a measured dose applicator.
NDC: 0168-0347-20
Store at 20°C to 25°C (68°F to 77°F), excursions permitted 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Risk of Skin Irritation and Flu-like Symptoms
Advise the patient to discontinue Terconazole Vaginal Cream, 0.8 % and not to retreat if skin irritation, fever, chills or flu-like symptoms are reported during use.
Important Administration Instructions
Inform patients using Terconazole Vaginal Cream, 0.8% of the following important administration instructions:
- Terconazole Vaginal Cream, 0.8% is for intravaginal use only. Avoid contact with eyes and inside the nose and mouth.
- Terconazole Vaginal Cream, 0.8% should be used for three days as directed, even if you feel better quickly.
- Do not use this Terconazole Vaginal Cream for any disorder other than the one for which it was prescribed.
- Terconazole Vaginal Cream, 0.8% should be used once a day as directed.
- If your sexual partner has itching, redness or discomfort in the genital area, tell your partner to consult a physician and that you are being treated for a yeast infection.
- Terconazole Vaginal Cream, 0.8% can be used during a menstrual period but external pads should be used until the medication treatment has been completed. Tampons may absorb the medication and should not be used. You may wish to wear an absorbent panty liner while using Terconazole Vaginal Cream, 0.8%.
- Don’t scratch the vaginal area. Scratching can cause more irritation and spread the infection.
- Wash hands before and after applying Terconazole Vaginal Cream, 0.8%.
Inform patients who are prone to vaginal yeast infections of the following additional instructions:
- Dry the genital area thoroughly after showering, bathing, or swimming. Change out of a wet bathing suit or damp exercise clothes as soon as possible. A dry environment is less likely to encourage the growth of yeast.
- Wipe from front to rear (away from the vagina) after a bowel movement.
- Don’t douche unless the healthcare provider specifically tells you to do so. Douching may disturb the balance of normal bacterial flora and the pH of secretions.
The brands listed are the registered trademarks of their respective owners and are not trademarks of Fougera Pharmaceuticals Inc.
Manufactured by:
E. FOUGERA & CO.
A division of Fougera Pharmaceuticals Inc.
Melville, New York 11747
-
PATIENT INFORMATION
PATIENT INFORMATION
TERCONAZOLE VAGINAL CREAM (ter kon ah zol)
Important: For use in the vagina only. Terconazole vaginal cream is not for use in the mouth or eyes.
Read the Patient Information that comes with Terconazole vaginal cream before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or treatment.
What is Terconazole vaginal cream?
Terconazole vaginal cream is prescription medicine used to treat vaginal yeast infections caused by a fungus called Candida in adult females.
It is not known if Terconazole vaginal cream is safe or effective in female children.
Do not use Terconazole vaginal cream if you:
are allergic to terconazole or any of the other ingredients of the cream. See the end of this leaflet for a complete list of ingredients in Terconazole vaginal cream.
Before using Terconazole vaginal cream, tell your healthcare provider about all of your medical conditions, including if you:
- have had an allergic reaction to another antifungal medicine.
- are pregnant or plan to become pregnant. It is not known if Terconazole vaginal cream will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Terconazole vaginal cream passes into your breast milk.
Tell your healthcare provider about all the medicines that you take including prescription and non-prescription medicines, vitamins and herbal supplements. Certain types of medicines can increase the chance of getting vaginal infections.
How should I use Terconazole vaginal cream?
- Use Terconazole vaginal cream exactly as your healthcare provider tells you to use it.
- Use 1 full applicator of Terconazole vaginal cream in the vagina each night at bedtime for 3 days in a row.
- Terconazole vaginal cream is not for use in the mouth or eyes.
- Do not stop using Terconazole vaginal cream before your healthcare provider tells you to, even if you feel better very quickly. Your infection may not clear up completely.
- See the end of this leaflet for detailed Instructions for Use about how to use Terconazole vaginal cream correctly.
- Avoid scratching the affected area. Scratching may cause more irritation and spread the infection.
- If your sexual partner has itching, redness, or discomfort in the genital area, tell your partner to see their healthcare provider and tell their healthcare provider that you are being treated for a yeast infection.
- Terconazole vaginal cream can be used during a menstrual period. You should not use tampons because they absorb the medicine. Use external pads until treatment with Terconazole vaginal cream has been completed.
- Follow your healthcare provider’s instructions about how to reduce your chances of getting vaginal yeast infections.
What are possible side effects with Terconazole vaginal cream?
Terconazole vaginal cream may cause serious side effects, including skin irritation and flu-like symptoms. Stop using Terconazole vaginal cream and do not use Terconazole vaginal cream again if you get any of the following symptoms during treatment:
- skin irritation
- fever, chills, or flu-like symptoms
If you have any of the symptoms listed above, tell your healthcare provider. Your healthcare provider may tell you to stop using Terconazole vaginal cream and prescribe a different medicine to treat your fungal infection.
Common side effects of Terconazole vaginal cream include:
- headache
- painful menstrual periods
- genital itching and burning
- stomach pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Terconazole vaginal cream. Ask your healthcare provider or pharmacist for more information.
Call your doctor for advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store Terconazole vaginal cream?
- Store Terconazole vaginal cream at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Terconazole vaginal cream and all medicines out of the reach of children.
General information about the safe and effective use of Terconazole vaginal cream
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflet. Do not use Terconazole vaginal cream for a condition for which it was not prescribed. Do not give Terconazole vaginal cream to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about Terconazole vaginal cream that is written for health professionals.
What are the ingredients in Terconazole vaginal cream?
Active ingredient: terconazole 0.8%
Inactive ingredients: butylated hydroxyanisole, cetyl alcohol, isopropyl myristate, polysorbate 60, polysorbate 80, propylene glycol, stearyl alcohol, and purified water.
Manufactured by:
E. FOUGERA & CO.
A division of Fougera Pharmaceuticals Inc.
Melville, New York 11747
For more information, call 1-800-645-9833.
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: September 2025
-
INSTRUCTIONS FOR USE
Instructions for Use
TERCONAZOLE VAGINAL CREAM (ter kon ah zol)
For vaginal use only. Do not put Terconazole vaginal cream in your eyes or mouth.
Be sure to read, understand, and follow the instructions below for how to use Terconazole vaginal cream, correctly.
Important Information
- Use Terconazole vaginal cream exactly as your healthcare provider tells you to use it.
- Use1 full applicator of terconazole vaginal cream in the vagina each night at bedtime for 3 days in a row.
- Do not stop using Terconazole vaginal cream before your healthcare provider tells you to, even if you feel better very quickly. Your infection may not clear up completely.
- If you have questions or if your vaginal symptoms do not go away, contact your healthcare provider.
How should I store terconazole vaginal cream?
- Store Terconazole vaginal cream at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Terconazole vaginal cream and all medicines out of the reach of children.
Manufactured by:
E. FOUGERA & CO.
A division of Fougera Pharmaceuticals Inc.
Melville, New York 11747
The brands listed are the registered trademarks of their respective owners and are not trademarks of Fougera Pharmaceuticals Inc.
This Instructions for Use has been approved by the U.S Food and Drug Administration Revised: 09/2025
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 0168-0347-20 Rx Only
Terconazole Vaginal Cream 0.8%
Tube and Applicator
NET WT 20 grams
Fougera
-
INGREDIENTS AND APPEARANCE
TERCONAZOLE VAGINAL CREAM 0.8%
terconazole creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0168-0347 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength terconazole (UNII: 0KJ2VE664U) (terconazole - UNII:0KJ2VE664U) terconazole 8 mg in 1 g Inactive Ingredients Ingredient Name Strength butylated hydroxyanisole (UNII: REK4960K2U) cetyl alcohol (UNII: 936JST6JCN) isopropyl myristate (UNII: 0RE8K4LNJS) polysorbate 60 (UNII: CAL22UVI4M) polysorbate 80 (UNII: 6OZP39ZG8H) propylene glycol (UNII: 6DC9Q167V3) stearyl alcohol (UNII: 2KR89I4H1Y) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0168-0347-20 20 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product 12/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021735 02/01/2005 Labeler - E. Fougera & Co. a division of Fougera Pharmaceuticals, LLC (043838424)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.