LONSURF- trifluridine and tipiracil tablet, film coated
LONSURF by
Drug Labeling and Warnings
LONSURF by is a Prescription medication manufactured, distributed, or labeled by Taiho Pharmaceutical Co., Ltd., Yuki Gosei Kogyo Co., Ltd., Taiho Pharmaceutical Co., Ltd. Saitama Plant, Taiho Pharmaceutical Co., Ltd. Kitajima Plant. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LONSURF safely and effectively. See full prescribing information for LONSURF.
LONSURF (trifluridine and tipiracil) tablets, for oral use
Initial U.S. Approval: 2015RECENT MAJOR CHANGES
INDICATIONS AND USAGE
LONSURF is a combination of trifluridine, a nucleoside metabolic inhibitor, and tipiracil, a thymidine phosphorylase inhibitor, indicated for the treatment of adult patients with:
- metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy. (1.1)
- metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neu-targeted therapy. (1.2)
DOSAGE AND ADMINISTRATION
- Recommended Dosage: 35 mg/m2/dose orally twice daily with food on Days 1 through 5 and Days 8 through 12 of each 28-day cycle. (2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Severe Myelosuppression: Obtain complete blood counts prior to and on Day 15 of each cycle. Withhold and resume at next lower LONSURF dosage as recommended. (2.1, 5.1)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.2, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions or laboratory abnormalities (≥10%) are anemia, neutropenia, fatigue/asthenia, nausea, thrombocytopenia, decreased appetite, diarrhea, vomiting, and pyrexia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Taiho Oncology, Inc. at 1-844-878-2446 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. (8.2)
- Geriatric Use: Grade 3 or 4 neutropenia and thrombocytopenia and Grade 3 anemia occurred more commonly in patients 65 years or older. (8.5)
- Hepatic Impairment: Do not initiate LONSURF in patients with baseline moderate or severe hepatic impairment. (8.7)
- Renal Impairment: Reduce LONSURF dose in patients with severe renal impairment. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Metastatic Colorectal Cancer
1.2 Metastatic Gastric Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications for Adverse Reactions
2.3 Recommended Dosage for Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Myelosuppression
5.2 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Metastatic Colorectal Cancer
14.2 Metastatic Gastric Cancer
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Metastatic Colorectal Cancer
LONSURF is indicated for the treatment of adult patients with metastatic colorectal cancer previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy.
1.2 Metastatic Gastric Cancer
LONSURF is indicated for the treatment of adult patients with metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neu-targeted therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of LONSURF is 35 mg/m2 up to a maximum of 80 mg per dose (based on the trifluridine component) orally twice daily with food on Days 1 through 5 and Days 8 through 12 of each 28-day cycle until disease progression or unacceptable toxicity. Round dose to the nearest 5 mg increment.
Instruct patients to swallow LONSURF tablets whole.
Instruct patients not to retake doses of LONSURF that are vomited or missed and to continue with the next scheduled dose.
LONSURF is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
Table 1 shows the calculated initial daily dose based on body surface area (BSA).Table 1 Recommended Dosage According to Body Surface Area (BSA)
BSA
(m2)Total daily
dose (mg)Dose (mg)
administered
twice dailyTablets per dose 15mg 20mg <1.07 70 35 1 1 1.07 - 1.22 80 40 0 2 1.23 - 1.37 90 45 3 0 1.38 - 1.52 100 50 2 1 1.53 - 1.68 110 55 1 2 1.69 - 1.83 120 60 0 3 1.84 - 1.98 130 65 3 1 1.99 - 2.14 140 70 2 2 2.15 - 2.29 150 75 1 3 ≥2.30 160 80 0 4 2.2 Dosage Modifications for Adverse Reactions
Obtain complete blood cell counts prior to and on Day 15 of each cycle [see Warnings and Precautions (5.1)].
Do not initiate the cycle of LONSURF until:
- Absolute neutrophil count (ANC) greater than or equal to 1,500/mm3 or febrile neutropenia is resolved
- Platelets greater than or equal to 75,000/mm3
- Grade 3 or 4 non-hematological adverse reactions are resolved to Grade 0 or 1
Within a treatment cycle, withhold LONSURF for any of the following:
- Absolute neutrophil count (ANC) less than 500/mm3 or febrile neutropenia
- Platelets less than 50,000/mm3
- Grade 3 or 4 non-hematologic adverse reaction
After recovery, resume LONSURF after reducing the dose by 5 mg/m2/dose from the previous dose, if the following occur:
- Febrile neutropenia
- Uncomplicated Grade 4 neutropenia (which has recovered to greater than or equal to 1,500/mm3) or thrombocytopenia (which has recovered to greater than or equal to 75,000/mm3) that results in more than 1 week delay in start of next cycle
- Non-hematologic Grade 3 or Grade 4 adverse reaction except for Grade 3 nausea and/or vomiting controlled by antiemetic therapy or Grade 3 diarrhea responsive to antidiarrheal medication
A maximum of 3 dose reductions are permitted. Permanently discontinue LONSURF in patients who are unable to tolerate a dose of 20 mg/m2 orally twice daily. Do not escalate LONSURF dosage after it has been reduced.
2.3 Recommended Dosage for Renal Impairment
Severe Renal Impairment
In patients with severe renal impairment [creatinine clearance (CLcr) of 15 to 29 mL/min as determined by the Cockcroft-Gault formula], the recommended dosage is 20 mg/m2 (based on the trifluridine component) orally twice daily with food on Days 1 through 5 and Days 8 through 12 of each 28-day cycle (Table 2) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. Reduce dose to 15 mg/m2 twice daily in patients with severe renal impairment who are unable to tolerate a dose of 20 mg/m2 twice daily (Table 2). Permanently discontinue LONSURF in patients who are unable to tolerate a dose of 15 mg/m2 twice daily.
Table 2 Recommended Dosage for Severe Renal Impairment According to BSA
* For a total daily dose of 50 mg, instruct patients to take 1 x 20-mg tablet in the morning and 2 x 15-mg tablets in the evening.
BSA (m2) Total daily
dose (mg)Dose (mg)
administered
twice dailyTablets per dose 15mg 20mg For a dose of 20 mg/m2 twice daily: < 1.14 40 20 0 1 1.14 – 1.34 50 25* 2 in the evening* 1 in the morning* 1.35 – 1.59 60 30 2 0 1.60 – 1.94 70 35 1 1 1.95 – 2.09 80 40 0 2 2.10 – 2.34 90 45 3 0 ≥ 2.35 100 50 2 1 For a dose of 15 mg/m2 twice daily: < 1.15 30 15 1 0 1.15 – 1.49 40 20 0 1 1.50 – 1.84 50 25* 2 in the evening* 1 in the morning* 1.85 – 2.09 60 30 2 0 2.10 – 2.34 70 35 1 1 ≥ 2.35 80 40 0 2 -
3 DOSAGE FORMS AND STRENGTHS
Tablets:
- 15 mg trifluridine/6.14 mg tipiracil: white, biconvex, round, film-coated, imprinted with ‘15’ on one side, and ‘102’ and ‘15 mg’ on the other side, in gray ink.
- 20 mg trifluridine/8.19 mg tipiracil: pale red, biconvex, round, film-coated, imprinted with ‘20’ on one side, and ‘102’ and ‘20 mg’ on the other side, in gray ink.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe Myelosuppression
In the 868 patients who received LONSURF in RECOURSE and TAGS, LONSURF caused severe and life-threatening myelosuppression (Grade 3-4) consisting of anemia (18%), neutropenia (38%), thrombocytopenia (5%) and febrile neutropenia (3%). Two patients (0.2%) died due to neutropenic infection/sepsis and four other patients (0.5%) died due to septic shock. A total of 12% of LONSURF-treated patients received granulocyte-colony stimulating factors.
Obtain complete blood counts prior to and on Day 15 of each cycle of LONSURF and more frequently as clinically indicated. Withhold LONSURF for severe myelosuppression and resume at the next lower dosage [see Dosage and Administration (2.2)].5.2 Embryo-Fetal Toxicity
Based on animal studies and its mechanism of action, LONSURF can cause fetal harm when administered to a pregnant woman. Trifluridine/tipiracil caused embryo-fetal lethality and embryo-fetal toxicity in pregnant rats when orally administered during gestation at dosage levels resulting in exposures lower than those achieved at the recommended dosage of 35 mg/m2 twice daily. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use an effective method of contraception during treatment with LONSURF and for at least 6 months after the final dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Severe Myelosuppression [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the WARNINGS AND PRECAUTIONS section and below reflect exposure to LONSURF at the recommended dose in 533 patients with metastatic colorectal cancer in RECOURSE and 335 patients with metastatic gastric cancer in TAGS. Among the 868 patients who received LONSURF, 11% were exposed for 6 months or longer and 1% were exposed for 12 months or longer. The most common adverse reactions or laboratory abnormalities (≥10%) are anemia, neutropenia, fatigue/asthenia, nausea, thrombocytopenia, decreased appetite, diarrhea, vomiting, and pyrexia.
Metastatic Colorectal Cancer
The safety of LONSURF was evaluated in RECOURSE, a randomized (2:1), double-blind, placebo-controlled trial in patients with previously treated metastatic colorectal cancer [see Clinical Studies (14.1)]. Patients received LONSURF 35 mg/m2/dose (n=533) or placebo (n=265) twice daily on Days 1 through 5 and Days 8 through 12 of each 28-day cycle. In RECOURSE, 12% of patients received LONSURF for more than 6 months and 1% of patients received LONSURF for more than 1 year.
The study population characteristics were: median age 63 years; 61% male; 57% White, 35% Asian, and 1% Black.
The most common adverse reactions or laboratory abnormalities (≥10% in incidence) in patients treated with LONSURF at a rate that exceeds the rate in patients receiving placebo were anemia, neutropenia, asthenia/fatigue, nausea, thrombocytopenia, decreased appetite, diarrhea, vomiting, abdominal pain, and pyrexia.
In RECOURSE, 3.6% of patients discontinued LONSURF for an adverse reaction and 14% of patients required a dose reduction. The most common adverse reactions or laboratory abnormalities leading to dose reduction were neutropenia, anemia, febrile neutropenia, fatigue, and diarrhea.
Tables 3 and 4 list the adverse reactions and laboratory abnormalities (graded using CTCAE v4.03), respectively, observed in RECOURSE.
Table 3 Adverse Reactions (≥5%) in Patients Receiving LONSURF and at a Higher Incidence (>2%) than in Patients Receiving Placebo in RECOURSE *No Grade 4 definition for nausea, abdominal pain, or fatigue in National Cancer Institute Common Terminology
†Incidence reflects 64 preferred terms in the Infections and Infestations system organ class.
Adverse Reactions LONSURF
(N=533)Placebo
(N=265)All Grades
(%)Grades 3-4*
(%)All Grades
(%)Grades 3-4*
(%)General Asthenia/fatigue 52 7 35 9 Pyrexia 19 1 14 <1 Gastrointestinal Nausea 48 2 24 1 Diarrhea 32 3 12 <1 Vomiting 28 2 14 <1 Abdominal pain 21 2 18 4 Stomatitis 8 <1 6 0 Metabolism and nutrition Decreased appetite 39 4 29 5 Infections† 27 6 16 5 Nervous system Dysgeusia 7 0 2 0 Skin and subcutaneous tissue Alopecia 7 0 1 0 Table 4 Laboratory Abnormalities in RECOURSE
* Worst Grade at least one grade higher than baseline, with percentages based on number of patients with post-baseline samples, which may be <533 (LONSURF) or 265 (placebo)
† One Grade 4 anemia adverse reaction based on clinical criteria was reported
Laboratory Parameter* LONSURF Placebo All Grades
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)Hematologic Anemia† 77 18 33 3 Neutropenia 67 38 1 0 Thrombocytopenia 42 5 8 <1 In RECOURSE, pulmonary emboli occurred more frequently in LONSURF-treated patients (2%) compared to no patients on placebo.
Metastatic Gastric Cancer
The safety of LONSURF was evaluated in TAGS, an international, randomized (2:1), double-blind, placebo-controlled trial in patients with metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma who were previously treated with at least 2 prior chemotherapy regimens for advanced disease [see Clinical Studies (14.2)]. Previous treatments must have included a fluoropyrimidine, a platinum, and either a taxane or irinotecan. Patients with HER2/neu-positive tumors must have received prior HER2/neu-targeted therapy, if available. Adjuvant chemotherapy could be counted as one prior regimen in patients who had recurrence during or within 6 months of completion of the adjuvant chemotherapy. Patients received LONSURF 35 mg/m2/dose (n=335) or placebo (n=168) twice daily on Days 1 through 5 and Days 8 through 12 of each 28-day cycle with best supportive care. In TAGS, 10% of patients received LONSURF for more than 6 months and 0.9% of patients received LONSURF for more than 1 year.
The study population characteristics were: median age 63 years (24 to 89 years); 73% male; 70% White, 16% Asian, and 1% Black.
The most common adverse reactions or laboratory abnormalities (≥10% in incidence) in patients treated with LONSURF at a rate that exceeds the rate in patients receiving placebo were neutropenia, anemia, nausea, decreased appetite, thrombocytopenia, vomiting, and diarrhea.
In TAGS, 13% of patients discontinued LONSURF for an adverse reaction and 11% of patients required a dose reduction. The most common adverse reactions or laboratory abnormalities leading to dose reduction were neutropenia, anemia, febrile neutropenia, and diarrhea.
Tables 5 and 6 list the adverse reactions and laboratory abnormalities (graded using CTCAE v4.03), respectively, observed in TAGS.
Table 5 Adverse Reactions (≥5%) in Patients Receiving LONSURF and at a Higher Incidence (>2%) than in Patients Receiving Placebo in TAGS *No Grade 4 definition for nausea or fatigue in NCI CTCAE, version 4.03.
†Incidence reflects 46 preferred terms in the Infections and Infestations system organ class.
Adverse Reactions LONSURF
(N=335)Placebo
(N=168)All Grades
(%)Grades 3-4*
(%)All Grades
(%)Grades 3-4*
(%)Gastrointestinal Nausea 37 3 32 3 Vomiting 25 4 20 2 Diarrhea 23 3 14 2 Metabolism and nutrition Decreased appetite 34 9 31 7 Infections† 23 5 16 5 Table 6 Laboratory Abnormalities in TAGS
* Worst Grade at least one Grade higher than baseline, with percent based on number of patients with post-baseline samples which may be <335 (LONSURF) or 168 (placebo)
† Anemia: No Grade 4 definition in CTCAE, v4.03
Laboratory Parameter* LONSURF Placebo All Grades
(%)Grades 3-4
(%)All Grades
(%)Grades 3-4
(%)Hematologic Neutropenia 66 38 4 0 Anemia† 63 19 38 7 Thrombocytopenia 34 6 9 0 In TAGS, pulmonary emboli occurred more frequently in LONSURF-treated patients (3.1%) compared to 1.8% for patients on placebo.
Additional Clinical Experience
Interstitial lung disease was reported in 15 (0.2%) patients, 3 of which were fatal, among approximately 7,000 patients exposed to LONSURF in clinical studies and clinical practice settings in Asia.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data and its mechanism of action [see Clinical Pharmacology (12.2)], LONSURF can cause fetal harm. LONSURF caused embryo-fetal lethality and embryo-fetal toxicity in pregnant rats when given during gestation at doses resulting in exposures lower than or similar to human exposures at the recommended clinical dose (see Data). There are no available data on LONSURF use in pregnant women. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
Trifluridine/tipiracil was administered orally once daily to female rats during organogenesis at dose levels of 15, 50, and 150 mg/kg [trifluridine (FTD) equivalent]. Decreased fetal weight was observed at FTD doses ≥50 mg/kg (approximately 0.33 times the FTD exposure at the clinical dose of 35 mg/m2 twice daily). At the FTD dose of 150 mg/kg (approximately 0.92 times the FTD exposure at the clinical dose of 35 mg/m2 twice daily) embryolethality and structural anomalies (kinked tail, cleft palate, ectrodactyly, anasarca, alterations in great vessels, and skeletal anomalies) were observed.
8.2 Lactation
Risk Summary
There are no data on the presence of trifluridine, tipiracil or its metabolites in human milk or its effects on the breastfed child or on milk production. In nursing rats, trifluridine and tipiracil or their metabolites were present in breast milk (see Data). Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with LONSURF and for 1 day following the final dose.
Radioactivity was excreted in the milk of nursing rats dosed with trifluridine/tipiracil containing 14C-FTD or 14C-tipiracil (TPI). Levels of FTD-derived radioactivity were as high as approximately 50% of the exposure in maternal plasma an hour after dosing with trifluridine/tipiracil and were approximately the same as those in maternal plasma for up to 12 hours following dosing. Exposure to TPI-derived radioactivity was higher in milk than in maternal plasma beginning 2 hours after dosing and continuing for at least 12 hours following administration of trifluridine/tipiracil.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating LONSURF [see Use in Specific Populations (8.1)].
Contraception
LONSURF can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Females
Advise females of reproductive potential to use effective contraception during treatment with LONSURF and for at least 6 months after the final dose.
Males
Because of the potential for genotoxicity, advise males with female partners of reproductive potential to use condoms during treatment with LONSURF and for at least 3 months after the final dose [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of LONSURF in pediatric patients have not been established.
Juvenile Animal Toxicity Data
Dental toxicity including whitening, breakage, and malocclusion (degeneration and disarrangement in the ameloblasts, papillary layer cells and odontoblasts) were observed in rats treated with trifluridine/tipiracil at doses ≥ 50 mg/kg (approximately 0.33 times the exposure at the clinical dose of 35 mg/m2 twice daily).
8.5 Geriatric Use
In RECOURSE and TAGS, 868 patients received LONSURF; 45% were 65 years of age or over, while 10% were 75 and over. No overall differences in effectiveness were observed in patients 65 or older versus younger patients. Patients 65 years of age or older who received LONSURF had a higher incidence of the following hematologic laboratory abnormalities compared to patients younger than 65 years: Grade 3 or 4 neutropenia (46% vs. 32%), Grade 3 anemia (22% vs. 16%), and Grade 3 or 4 thrombocytopenia (7% vs. 4%).
8.6 Renal Impairment
No dose adjustment is recommended for patients with mild or moderate renal impairment (CLcr of 30 to 89 mL/min as determined by the Cockcroft-Gault formula). Reduce the dose of LONSURF for patients with severe renal impairment (CLcr of 15 to 29 mL/min) [see Dosage and Administration (2.3)]. The pharmacokinetics of trifluridine and tipiracil have not been studied in patients with end stage renal disease.
8.7 Hepatic Impairment
No adjustment to the starting dosage of LONSURF is recommended for patients with mild hepatic impairment. Do not initiate LONSURF in patients with baseline moderate or severe (total bilirubin >1.5 times ULN and any AST) hepatic impairment [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
LONSURF contains trifluridine and tipiracil hydrochloride at a molar ratio of 1:0.5.
Trifluridine
Trifluridine, a nucleoside metabolic inhibitor, is described chemically as 2’-deoxy-5-(trifluoromethyl) uridine and has the following structural formula:
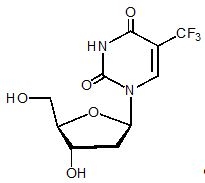
Trifluridine has a molecular formula C10H11F3N2O5 and a molecular weight of 296.20. Trifluridine is a white crystalline powder, soluble in water, ethanol, 0.01 mol/L hydrochloric acid, 0.01 mol/L sodium hydroxide solution; freely soluble in methanol, acetone; sparingly soluble in 2-propanol, acetonitrile; slightly soluble in diethyl ether; and very slightly soluble in isopropyl ether.
Tipiracil hydrochloride
Tipiracil hydrochloride, a thymidine phosphorylase inhibitor, is described chemically as 5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]pyrimidine-2,4-(1H,3H)-dione monohydrochloride or 2,4(1H,3H)-Pyrimidinedione, 5-chloro-6-[(2-imino-1-pyrrolidinyl)methyl]-, hydrochloride (1:1) and has the following structural formula:
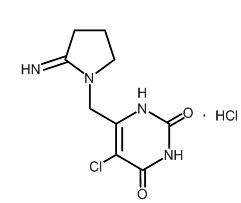
Tipiracil hydrochloride has a molecular formula C9H11ClN4O2HCl and a molecular weight of 279.12. Tipiracil hydrochloride is a white crystalline powder, soluble in water, 0.01 mol/L hydrochloric acid, and 0.01 mol/L sodium hydroxide; slightly soluble in methanol; very slightly soluble in ethanol; and practically insoluble in acetonitrile, 2-propanol, acetone, diisopropyl ether, and diethyl ether.
LONSURF (trifluridine and tipracil) tablets for oral use contain 15 mg of trifluridine and 6.14 mg of tipiracil equivalent to 7.065 mg of tipiracil hydrochloride or 20 mg of trifluridine and 8.19 mg of tipiracil equivalent to 9.420 mg of tipiracil hydrochloride.
LONSURF tablets contain the following inactive ingredients: lactose monohydrate, pregelatinized starch, stearic acid, hypromellose, polyethylene glycol, titanium dioxide, ferric oxide, and magnesium stearate. The tablets are imprinted with ink containing shellac, ferric oxide red, ferric oxide yellow, titanium dioxide, FD&C Blue No. 2 Aluminum Lake, carnauba wax, and talc.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
LONSURF consists of a thymidine-based nucleoside analog, trifluridine, and the thymidine phosphorylase inhibitor, tipiracil, at a molar ratio 1:0.5 (weight ratio, 1:0.471). Inclusion of tipiracil increases trifluridine exposure by inhibiting its metabolism by thymidine phosphorylase.
Following uptake into cancer cells, trifluridine is incorporated into DNA, interferes with DNA synthesis and inhibits cell proliferation. Trifluridine/tipiracil demonstrated anti-tumor activity against KRAS wild-type and mutant human colorectal cancer xenografts in mice.
12.2 Pharmacodynamics
Cardiac Electrophysiology
LONSURF administered to 42 patients with advanced solid tumors at the recommended dosage had no large effect (i.e. >20 ms) in the mean QTc interval when compared to placebo and no exposure-QT relationship was identified. Two of 42 patients (4.8%) had QTc >500 msec and 2.4% had a QTc increase from baseline >60 msec.
12.3 Pharmacokinetics
After twice daily dosing of LONSURF, systemic exposure (AUC) of trifluridine increased more than dose-proportionally over the dose range of 15 mg/m2 (0.43 times the recommended dose) to 35 mg/m2.
The accumulation of trifluridine was 3-fold for AUC0-12hr and 2-fold for Cmax at steady state while no accumulation was observed for tipiracil.
Administration of a single dose of LONSURF 35 mg/m2 increased the mean AUC0-last of trifluridine by 37-fold and Cmax by 22-fold with reduced variability compared to administration of a single dose of trifluridine 35 mg/m2 alone.
Absorption
Following a single oral administration of LONSURF at 35 mg/m2 in patients with cancer, the mean time to peak plasma concentration (Tmax) of trifluridine was around 2 hours.
Food Effect
A standardized high-fat, high-calorie meal decreased trifluridine Cmax, tipiracil Cmax and AUC by approximately 40%, but did not change trifluridine AUC compared to those in a fasting state in patients with cancer following administration of a single dose of LONSURF 35 mg/m2.
Distribution
Trifluridine mainly binds to human serum albumin. The in vitro protein binding of trifluridine in human plasma is >96%, independent of drug concentration and presence of tipiracil. Plasma protein binding of tipiracil is below 8%.
Elimination
After administration of LONSURF 35 mg/m2, the mean elimination half-life (t1/2) of trifluridine was 1.4 hours and of tipiracil was 2.1 hours after a single dose. The mean elimination half-life at steady state of trifluridine was 2.1 hours and of tipiracil was 2.4 hours.
Metabolism
Trifluridine and tipiracil are not metabolized by cytochrome P450 (CYP) enzymes. Trifluridine is mainly eliminated by metabolism via thymidine phosphorylase to form an inactive metabolite, 5-(trifluoromethyl) uracil (FTY). No other major metabolites were detected in plasma or urine.
Excretion
After single oral administration of LONSURF (60 mg) with [14C]-trifluridine, the total cumulative excretion of radioactivity was 60% of the administered dose. The majority of recovered radioactivity was eliminated into urine (55% of the dose) as FTY and trifluridine glucuronide isomers within 24 hours and the excretion into feces and expired air was <3% for both. The unchanged trifluridine was <3% of administered dose recovered in the urine and feces.
After single oral administration of LONSURF (60 mg) with [14C]-tipiracil hydrochloride, recovered radioactivity was 77% of the dose, which consisted of 27% urinary excretion and 50% fecal excretion. Tipiracil was the major component and 6-HMU was the major metabolite in urine, and feces.
Specific Populations
Based on the population pharmacokinetic analysis, there is no clinically relevant effect of age, sex, or race (White or Asian) on the pharmacokinetics of trifluridine or tipiracil.
Patients with Renal Impairment
In a dedicated renal impairment study, all patients received LONSURF 35 mg/m2 twice daily except for patients with severe renal impairment who received 20 mg/m2 twice daily. Mild renal impairment (CLcr of 60 to 89 mL/min as determined by the Cockcroft-Gault formula) had no clinically important effect on steady-state AUC0-last of trifluridine and tipiracil. Moderate renal impairment (CLcr of 30 to 59 mL/min) increased steady-state AUC0-last of trifluridine by 56% and tipiracil by 139% compared to normal renal function (CLcr ≥ 90 mL/min). Severe renal impairment (CLcr of 15 to 29 mL/min) increased the dose-normalized steady-state AUC0-last of trifluridine by 140% and tipiracil by 614% compared to normal renal function. The pharmacokinetics of trifluridine and tipiracil have not been studied in patients with end stage renal disease.
Patients with Hepatic Impairment
No clinically important differences in the mean exposures of trifluridine and tipiracil were observed between patients with mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin <1 to 1.5 times ULN and any AST) to moderate hepatic impairment (total bilirubin >1.5 to 3 times ULN and any AST) and patients with normal hepatic function (total bilirubin and AST ≤ ULN); however, 5 of 6 patients with moderate hepatic impairment experienced Grade 3 or 4 increased bilirubin levels. The pharmacokinetics of trifluridine and tipiracil have not been studied in patients with severe hepatic impairment [see Dosage Modifications (2.2), Use in Specific Populations (8.6)].
Drug Interaction Studies
In vitro studies indicated that trifluridine, tipiracil, and FTY did not inhibit the CYP enzymes and had no inductive effect on CYP1A2, CYP2B6, or CYP3A4/5.
In vitro studies indicated that trifluridine was not an inhibitor of or substrate for human uptake and efflux transporters.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies evaluating the carcinogenic potential of trifluridine/tipiracil in animals have been performed. Trifluridine/tipiracil was genotoxic in a reverse mutation test in bacteria, a chromosomal aberration test in mammalian-cultured cells, and a micronucleus test in mice.
Animal studies did not indicate an effect of trifluridine/tipiracil on male fertility in rats. Dose-related increases in the corpus luteum count and implanted embryo count were observed, but female fertility was not affected.
-
14 CLINICAL STUDIES
14.1 Metastatic Colorectal Cancer
The efficacy of LONSURF was evaluated in RECOURSE (NCT01607957), an international, randomized, double-blind, placebo-controlled study conducted in patients with previously treated metastatic colorectal cancer (mCRC). Key eligibility criteria included prior treatment with at least 2 lines of standard chemotherapy for metastatic CRC, ECOG performance status (PS) 0-1, absence of brain metastasis, and absence of ascites requiring drainage in the past four weeks. Patients were randomized 2:1 to receive LONSURF 35 mg/m2 or matching placebo orally twice daily after meals on Days 1-5 and 8-12 of each 28-day cycle until disease progression or unacceptable toxicity. Randomization was stratified by KRAS status (wild-type vs. mutant), time since diagnosis of first metastasis (<18 months vs. ≥ 18 months), and region (Japan vs. US, Europe and Australia). The major efficacy outcome measure was overall survival (OS) and an additional efficacy outcome measure was progression-free survival (PFS).
A total of 800 patients were randomized to LONSURF (N=534) with best supportive care (BSC) or matching placebo (N=266) plus BSC. The median age was 63 years, 61% were male, 58% and 35% were White and Asian respectively, and all patients had baseline ECOG PS of 0 or 1. The primary site of disease was colon (62%) or rectum (38%). KRAS status was wild-type (49%) or mutant (51%) at study entry. All patients received prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy. All but one patient received bevacizumab and all but two patients with KRAS wild-type tumors received panitumumab or cetuximab.
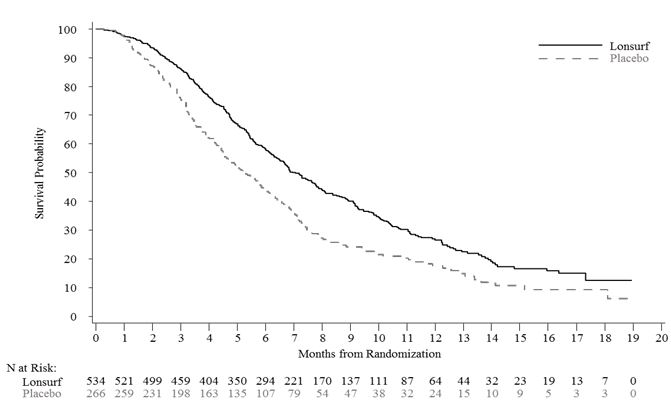
Efficacy results are summarized in Table 7 and Figure 1.
Table 7 Efficacy Results from RECOURSE
a Kaplan-Meier estimates b Methodology of Brookmeyer and Crowley c Stratified log-rank test (strata: KRAS status, time since diagnosis of first metastasis, region), 2-sided LONSURF
(N=534)Placebo
(N=266)Overall Survival Number of deaths, N (%) 364 (68) 210 (79) Median OS (months)a (95% CI)b 7.1 (6.5, 7.8) 5.3 (4.6, 6.0) Hazard ratio (95% CI) 0.68 (0.58, 0.81) p-valuec <0.001 Progression-Free Survival Number of events, N (%) 472 (88) 251 (94) Hazard ratio (95% CI) 0.47 (0.40, 0.55) p-valuec <0.001 Figure 1 Kaplan-Meier Curves of Overall Survival in RECOURSE
14.2 Metastatic Gastric Cancer
The efficacy of LONSURF was evaluated in TAGS (NCT02500043), an international, randomized, double-blind, placebo-controlled study in patients with metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma previously treated with at least 2 prior regimens for advanced disease. Previous treatments must have included a fluoropyrimidine, a platinum, and either a taxane or irinotecan. Patients with HER2/neu-positive tumors must have received prior HER2/neu-targeted therapy, if available. Adjuvant chemotherapy could be counted as one prior regimen in patients who had recurrence during or within 6 months of completion of the adjuvant chemotherapy. Other key eligibility criteria included ECOG performance status (PS) 0 or 1. Patients were randomized 2:1 to receive LONSURF 35 mg/m2 orally twice daily on Days 1-5 and 8-12 of each 28-day cycle with best supportive care (BSC) or matching placebo with BSC until disease progression or unacceptable toxicity. Randomization was stratified by ECOG PS at baseline (0 vs. 1), prior ramucirumab (yes vs. no), and geographic region (Japan vs. rest of world). The major efficacy outcome measure was OS and an additional outcome measure was PFS.
A total of 507 patients were randomized to LONSURF (N=337) or placebo (N=170). The median age was 63 years, 73% were male, 70% and 16% were White and Asian respectively, and 38% had a baseline ECOG PS of 0. Seventy-one percent of patients had gastric tumors, 29% had GEJ tumors, and two patients had gastric/GEJ tumors. All patients received platinum-based chemotherapy, 99% received fluoropyrimidine-based therapy, 91% received a taxane, 55% received irinotecan, and 33% received ramucirumab. The HER2 status was negative in 62%, positive in 19%, and unknown in 20% of patients. Among the 94 patients with HER2 positive tumors, 89% received prior anti-HER2 therapy.
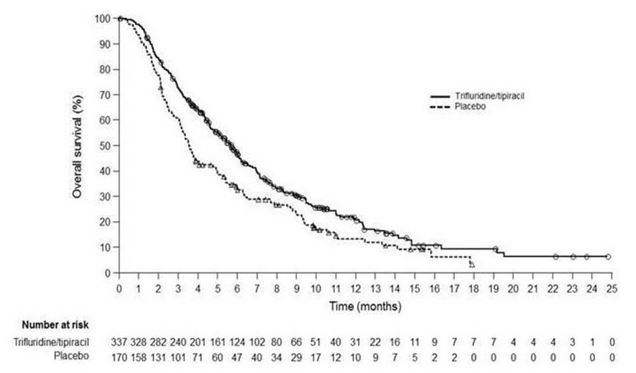
Efficacy results are summarized in Table 8 and Figure 2.
Table 8 Efficacy Results from TAGS
a Kaplan-Meier estimates b Methodology of Brookmeyer and Crowley c Stratified log-rank test (strata: ECOG PS, prior ramucirumab treatment, region), 2-sided LONSURF
(N=337)Placebo
(N=170)Overall Survival Number of deaths, N (%) 244 (72) 140 (82) Median OS (months)a (95% CI)b 5.7 (4.8, 6.2) 3.6 (3.1, 4.1) Hazard ratio (95% CI) 0.69 (0.56, 0.85) p-valuec 0.0006 Progression-Free Survival Number of events, N (%) 287 (85) 156 (92) Hazard ratio (95% CI) 0.56 (0.46, 0.68) p-valuec <0.0001 Figure 2 Kaplan-Meier Curves of Overall Survival in TAGS
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
LONSURF 15 mg/6.14 mg tablets are supplied as white, biconvex, round, film-coated tablet, imprinted with ‘15’ on one side, and ‘102’ and ’15 mg’ on the other side, in gray ink. The tablets are packaged in HDPE bottles with child resistant closures in the following presentations:
- 20 count: NDC: 64842-1025-1
- 40 count: NDC: 64842-1025-2
- 60 count: NDC: 64842-1025-3
LONSURF 20 mg/8.19 mg tablets are supplied as pale red, biconvex, round, film-coated tablet, imprinted with ‘20’ on one side, and ‘102’ and ‘20 mg’ on the other side, in gray ink. The tablets are packaged in HDPE bottles with child resistant closures in the following presentations:
- 20 count: NDC: 64842-1020-1
- 40 count: NDC: 64842-1020-2
- 60 count: NDC: 64842-1020-3
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted from 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
LONSURF is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
If stored outside of original bottle, discard after 30 days.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Severe Myelosuppression
Advise patients to immediately contact their healthcare provider if they experience signs or symptoms of infection and advise patients to keep all appointments for blood tests [see Warnings and Precautions (5.1)].
Gastrointestinal Toxicity
Advise patients to contact their healthcare provider for severe or persistent nausea, vomiting, diarrhea, or abdominal pain [see Adverse Reactions (6.1)].
Administration Instructions
Advise patients that LONSURF is available in two strengths and they may receive both strength tablets to provide the prescribed dosage.
Advise patients to take LONSURF with food [see Dosage and Administration (2.1)].
Advise patients that anyone else who handles their medication should wear gloves [see References (15)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to the fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.2), Use in Specific Populations (8.3)].
Advise female patients of reproductive potential to use effective contraception during treatment with LONSURF and for at least 6 months after the final dose [see Warnings and Precautions (5.2), Use in Specific Populations (8.3)].
Advise males with female partners of reproductive potential to use condoms during treatment with LONSURF and for at least 3 months after the final dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with LONSURF and for 1 day following the final dose [see Use in Specific Populations (8.2)].
Manufactured by: Taiho Pharmaceutical Co., Ltd., Japan
Manufactured for: Taiho Oncology, Inc., Princeton, NJ 08540 USALONSURF is a registered trademark of Taiho Pharmaceutical Co., Ltd used under license by
Taiho Oncology, Inc. -
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 2/2019 PATIENT INFORMATION
LONSURF® (LON-serf)
(trifluridine and tipiracil)
tabletsWhat is the most important information I should know about LONSURF?
Your healthcare provider should do blood tests before you receive LONSURF, at day 15 during treatment with LONSURF, and as needed to check your blood cell counts.
LONSURF may cause serious side effects, including:
Low blood cell counts. Low blood counts are common with LONSURF and can sometimes be severe and life-threatening. LONSURF can cause a decrease in your white blood cells, red blood cells, and platelets. Low white blood cells can make you more likely to get serious infections that could lead to death. Your healthcare provider may:- lower your dose of LONSURF or stop LONSURF if you have low white blood cell or low platelet counts.
- fever
- chills
- body aches
What is LONSURF?
LONSURF is a prescription medicine used to treat people with- colorectal cancer that has spread to other parts of the body and who have been previously treated or cannot receive certain chemotherapy medicines.
- a kind of stomach cancer called gastric cancer including adenocarcinoma of the gastroesophageal junction that has spread to other parts of the body and who have been previously treated or cannot receive certain chemotherapy medications.
It is not known if LONSURF is safe and effective in children.Before you take LONSURF, tell your healthcare provider about all of your medical conditions, including if you: - have kidney or liver problems
- are pregnant or plan to become pregnant. LONSURF can harm your unborn baby.
For females who can become pregnant:- Your healthcare provider will verify your pregnancy status before you start treatment with LONSURF.
- You should use effective birth control during treatment with LONSURF and for at least 6 months after your last dose of LONSURF.
- Tell your healthcare provider right away if you become pregnant.
- You should use a condom during sex with female partners who are able to become pregnant during your treatment with LONSURF and for 3 months after your last dose of LONSURF. Tell your healthcare provider right away if your partner becomes pregnant while you are taking LONSURF.
- are breastfeeding or plan to breastfeed. It is not known if LONSURF passes into breast milk. Do not breastfeed during treatment with LONSURF and for one day after your last dose of LONSURF.
How should I take LONSURF? - Take LONSURF exactly as your healthcare provider tells you.
LONSURF comes in two strengths. Your healthcare provider may prescribe both strengths for your prescribed dose. - Take LONSURF 2 times a day with food.
- Swallow LONSURF tablets whole.
- Your caregiver should wear gloves when handling LONSURF tablets.
- If you miss a dose of LONSURF, do not take additional doses to make up for the missed dose. Call your healthcare provider for instructions about what to do for a missed dose.
- Wash your hands after handling the LONSURF tablets.
What are the possible side effects of LONSURF?
LONSURF may cause serious side effects, including:- See “What is the most important information I should know about LONSURF?”
The most common side effects of LONSURF include:- Tiredness (fatigue, weakness)
- nausea
- decreased appetite
- diarrhea
- vomiting
- abdominal pain
- fever
These are not all of the possible side effects of LONSURF. For more information, ask your healthcare provider.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store LONSURF? - Store LONSURF at room temperature between 68°F and 77°F (20°C and 25°C).
- If you store LONSURF outside of the original bottle, throw away (dispose of) any unused LONSURF tablets after 30 days.
- Talk to your healthcare provider about how to safely dispose of LONSURF.
General information about the safe and effective use of LONSURF
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LONSURF for a condition for which it was not prescribed. Do not give LONSURF to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about LONSURF that is written for health professionals.What are the ingredients in LONSURF?
Active ingredients: trifluridine and tipiracil hydrochloride
Other ingredients: lactose monohydrate, pregelatinized starch, stearic acid, hypromellose, polyethylene glycol, titanium dioxide, ferric oxide (20 mg tablet only), and magnesium stearate
Imprinting ink: shellac, ferric oxide red, ferric oxide yellow, titanium dioxide, FD&C Blue No. 2 Aluminum Lake, carnauba wax, and talc.
Manufactured by: Taiho Pharmaceutical Co., Ltd., Japan
Manufactured for: Taiho Oncology, Inc., Princeton, NJ 08540 USA
LONSURF is a registered trademark of Taiho Oncology, Inc. For more information, go to www.Lonsurf.com or call 1-844-878-2446.
-
PRINCIPAL DISPLAY PANEL
NDC: 64842-1025-1
Lonsurf®
(trifluridine and tipiracil*) tablets15 mg/6.14 mg*
20 tablets
Rx only
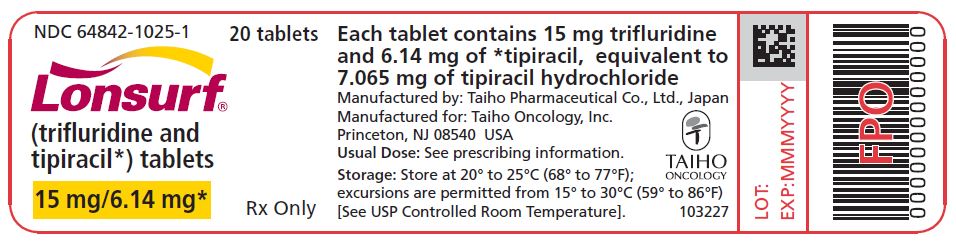
NDC: 64842-1025-1
Lonsurf®
(trifluridine and tipiracil*) tablets15 mg/6.14 mg*
For oral administraion
20 tabletsRx only
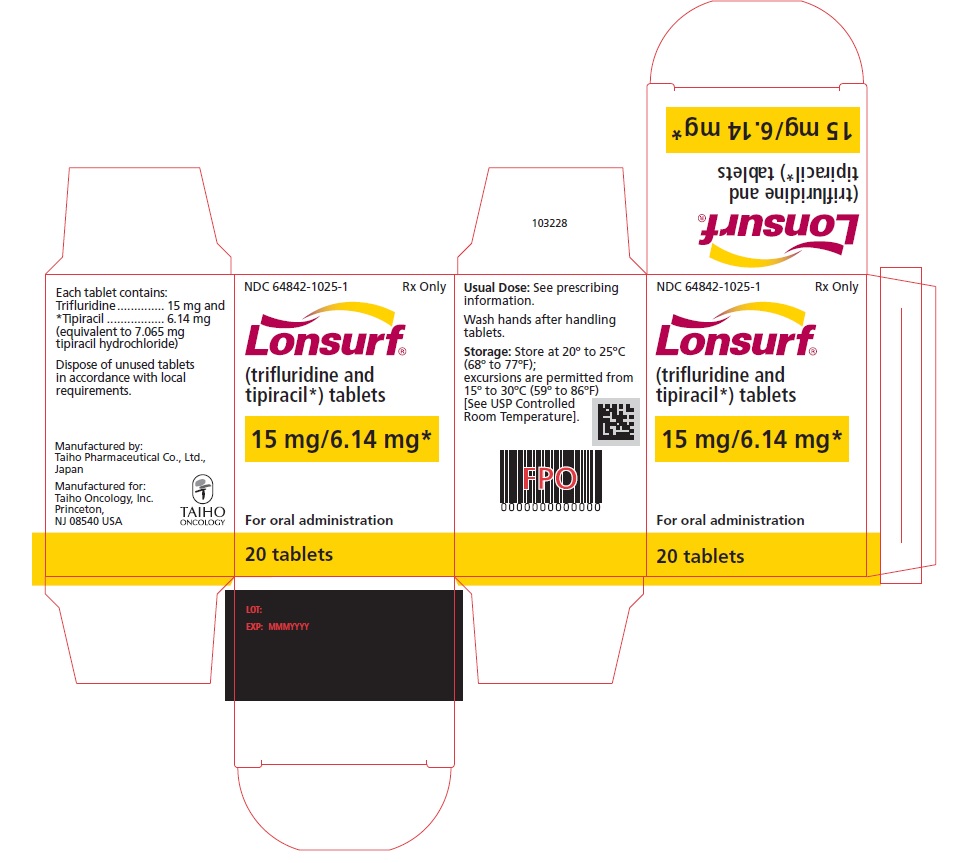
NDC: 64842-1025-2
Lonsurf®
(trifluridine and tipiracil*) tablets15 mg/6.14 mg*
40 tablets
Rx only

NDC: 64842-1025-2
Lonsurf®
(trifluridine and tipiracil*) tablets15 mg/6.14 mg*
For oral administraion
40 tabletsRx only
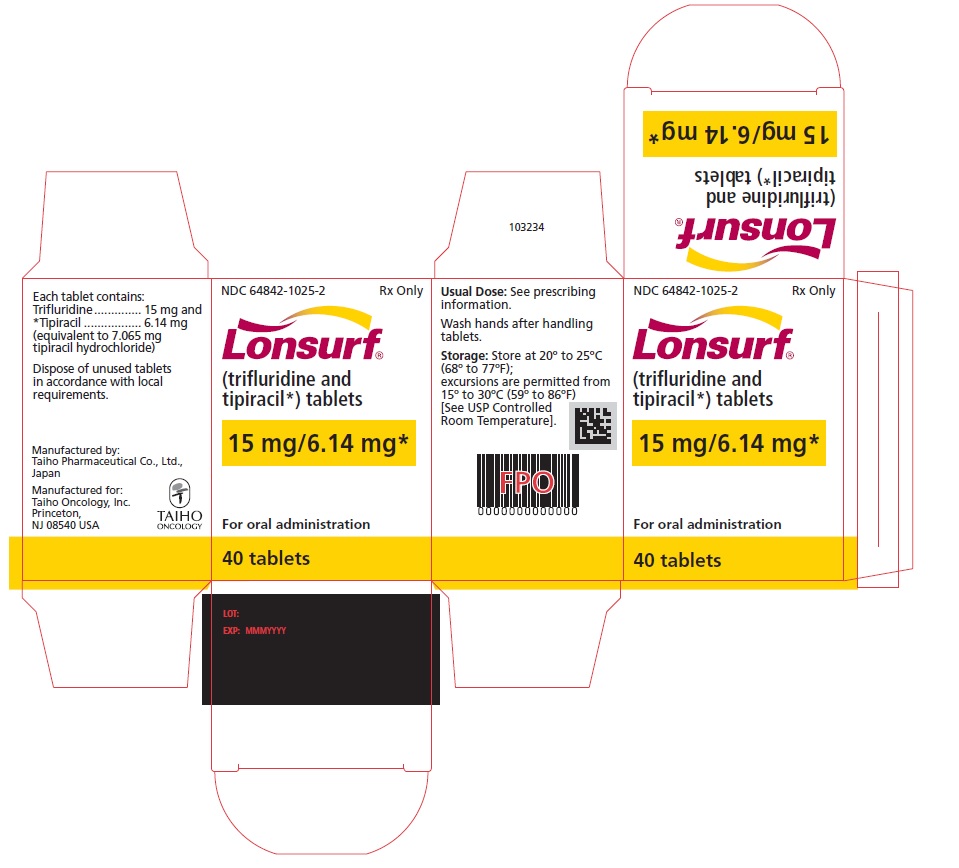
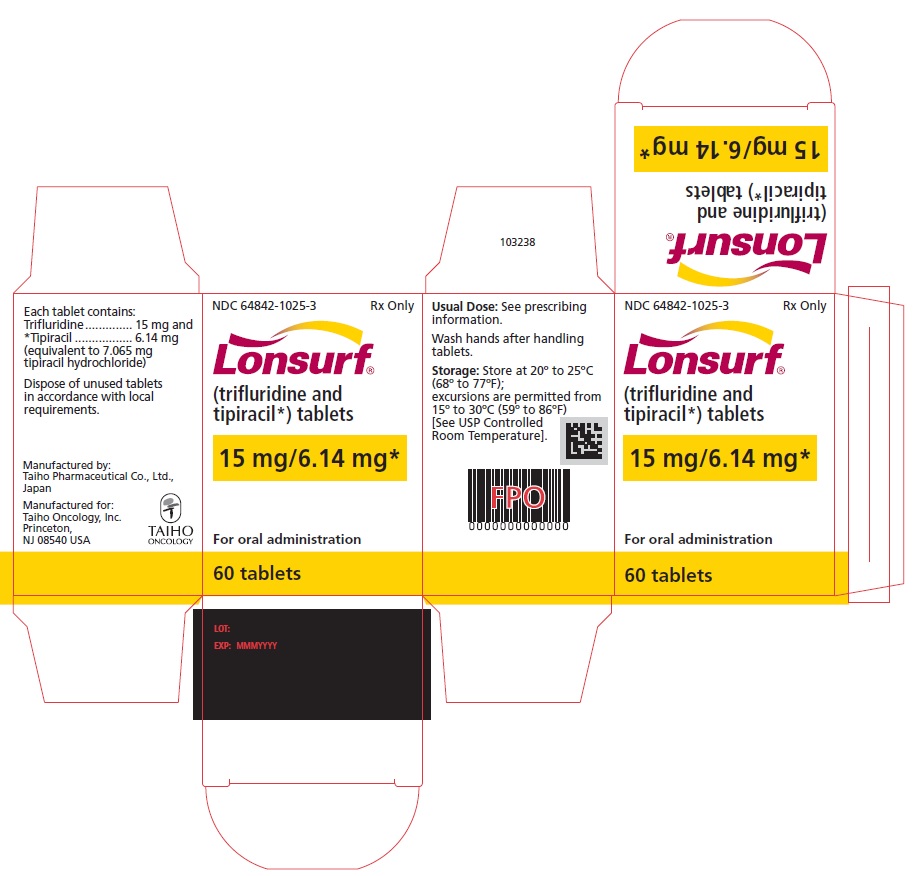
NDC: 64842-1025-3
Lonsurf®
(trifluridine and tipiracil*) tablets15 mg/6.14 mg*
60 tablets
Rx only

NDC: 64842-1025-3
Lonsurf®
(trifluridine and tipiracil*) tablets15 mg/6.14 mg*
For oral administraion
60 tabletsRx only
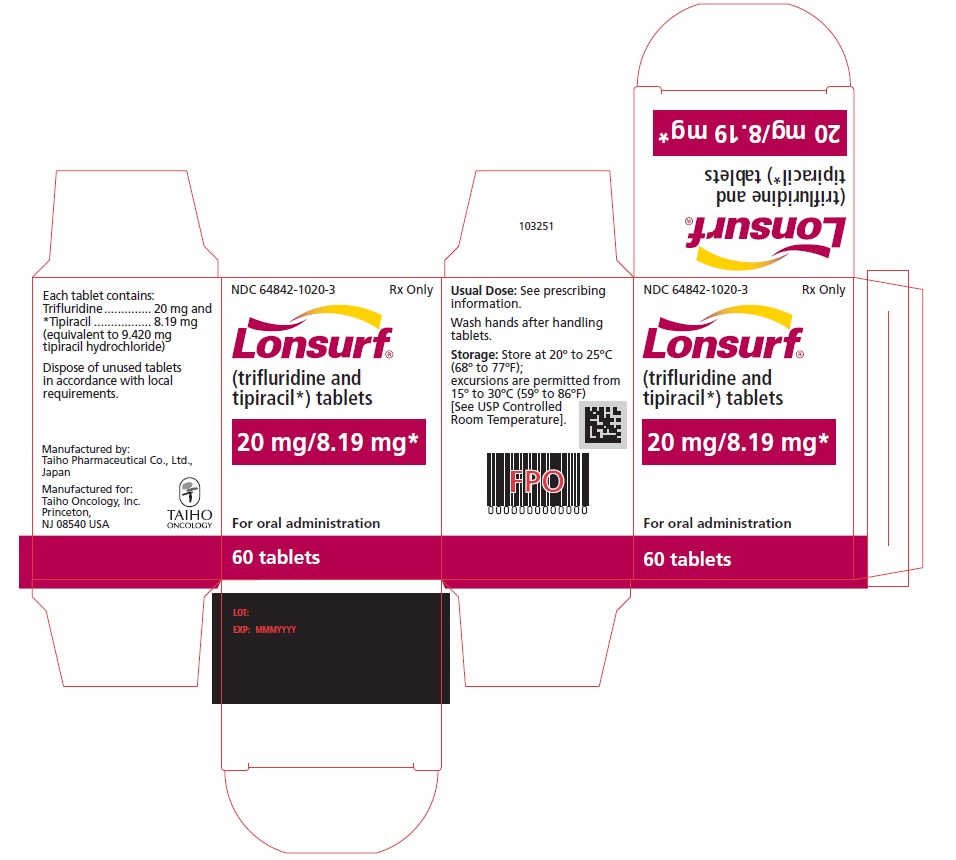
NDC: 64842-1020-1
Lonsurf®
(trifluridine and tipiracil*) tablets20 mg/8.19 mg*
20 tablets
Rx only

NDC: 64842-1020-1
Lonsurf®
(trifluridine and tipiracil*) tablets20 mg/8.19 mg*
For oral administraion
20 tabletsRx only
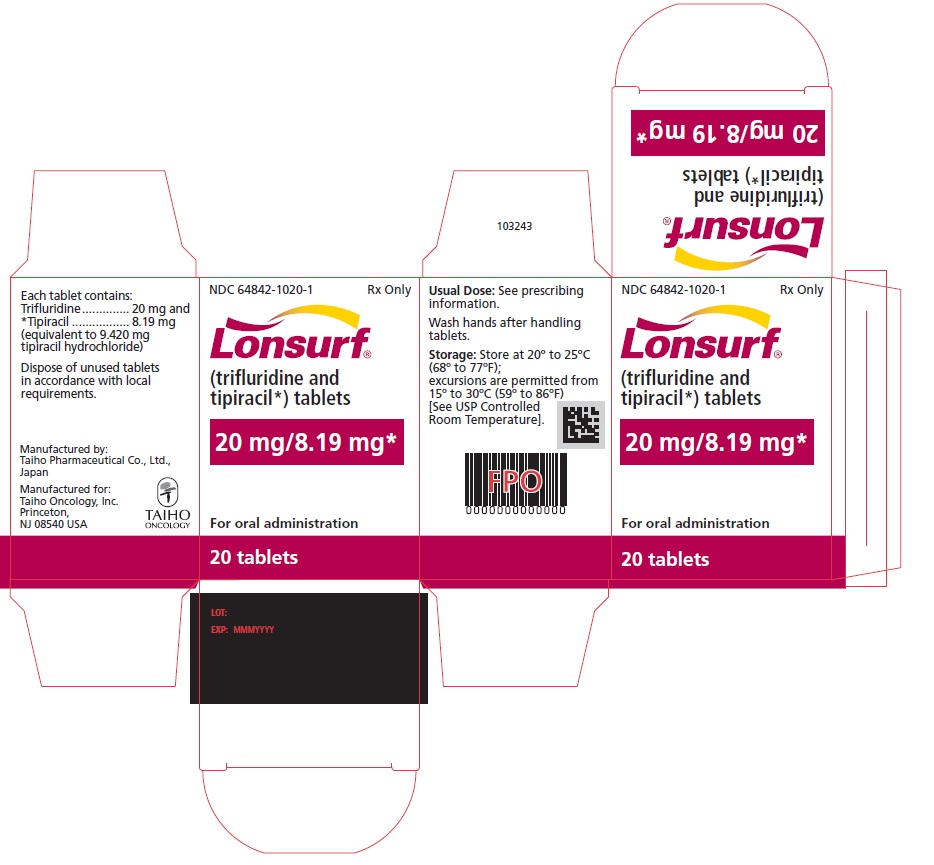
NDC: 64842-1020-2
Lonsurf®
(trifluridine and tipiracil*) tablets20 mg/8.19 mg*
40 tablets
Rx only

NDC: 64842-1020-2
Lonsurf®
(trifluridine and tipiracil*) tablets20 mg/8.19 mg*
For oral administraion
40 tabletsRx only
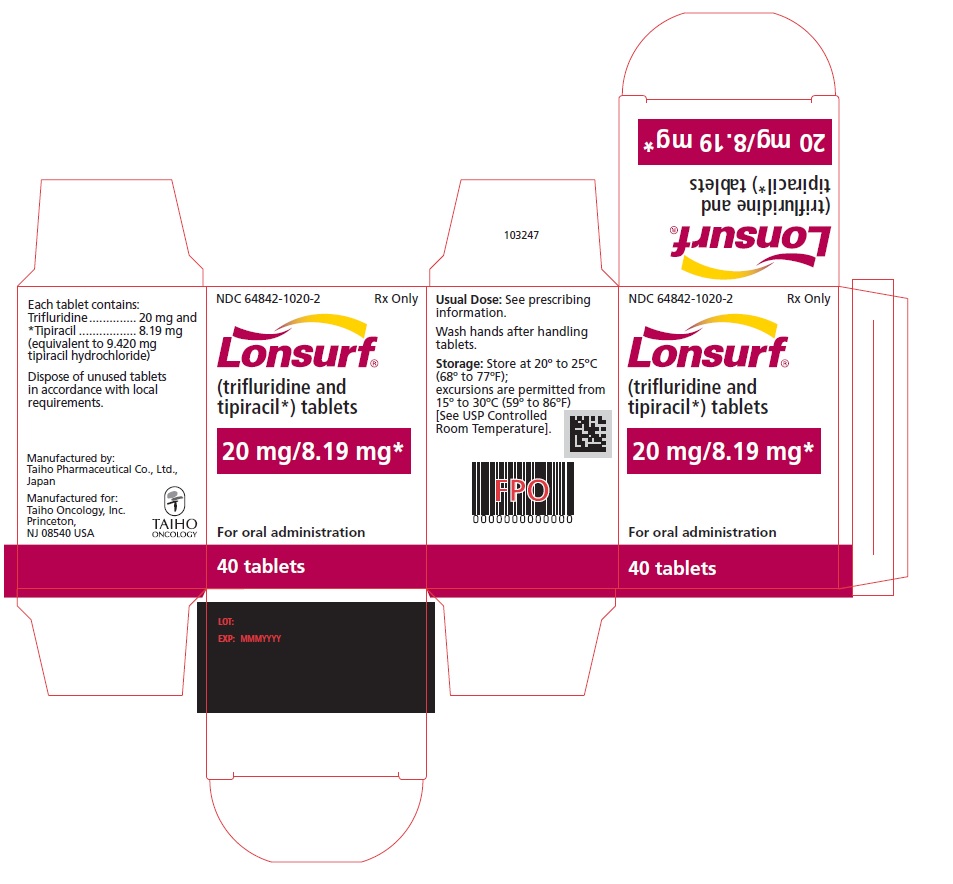
NDC: 64842-1020-3
Lonsurf®
(trifluridine and tipiracil*) tablets20 mg/8.19 mg*
60 tablets
Rx only

NDC: 64842-1020-3
Lonsurf®
(trifluridine and tipiracil*) tablets20 mg/8.19 mg*
For oral administraion
60 tabletsRx only
-
INGREDIENTS AND APPEARANCE
LONSURF
trifluridine and tipiracil tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64842-1025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIFLURIDINE (UNII: RMW9V5RW38) (TRIFLURIDINE - UNII:RMW9V5RW38) TRIFLURIDINE 15 mg TIPIRACIL HYDROCHLORIDE (UNII: 4H59KLQ0A4) (TIPIRACIL - UNII:NGO10K751P) TIPIRACIL 6.14 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) 90.735 mg STARCH, CORN (UNII: O8232NY3SJ) 6.000 mg STEARIC ACID (UNII: 4ELV7Z65AP) 1.200 mg HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) 2.100 mg POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) 0.300 mg TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.300 mg MAGNESIUM STEARATE (UNII: 70097M6I30) 0.003 mg ALCOHOL (UNII: 3K9958V90M) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) Product Characteristics Color WHITE Score no score Shape ROUND (biconvex) Size 7mm Flavor Imprint Code 15;102;15;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64842-1025-1 20 in 1 BOTTLE; Type 0: Not a Combination Product 10/07/2015 2 NDC: 64842-1025-2 40 in 1 BOTTLE; Type 0: Not a Combination Product 11/11/2015 3 NDC: 64842-1025-3 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207981 09/22/2015 LONSURF
trifluridine and tipiracil tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64842-1020 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIFLURIDINE (UNII: RMW9V5RW38) (TRIFLURIDINE - UNII:RMW9V5RW38) TRIFLURIDINE 20 mg TIPIRACIL HYDROCHLORIDE (UNII: 4H59KLQ0A4) (TIPIRACIL - UNII:NGO10K751P) TIPIRACIL 8.19 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) 120.980 mg STARCH, CORN (UNII: O8232NY3SJ) 8.000 mg STEARIC ACID (UNII: 4ELV7Z65AP) 1.600 mg HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) 2.800 mg POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) 0.400 mg TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.400 mg FERRIC OXIDE RED (UNII: 1K09F3G675) 0.040 mg MAGNESIUM STEARATE (UNII: 70097M6I30) 0.004 mg ALCOHOL (UNII: 3K9958V90M) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) Product Characteristics Color RED (pale red) Score no score Shape ROUND (biconvex) Size 8mm Flavor Imprint Code 20;102;20;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64842-1020-1 20 in 1 BOTTLE; Type 0: Not a Combination Product 10/07/2015 2 NDC: 64842-1020-2 40 in 1 BOTTLE; Type 0: Not a Combination Product 11/11/2015 3 NDC: 64842-1020-3 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/21/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207981 09/22/2015 Labeler - Taiho Pharmaceutical Co., Ltd. (690548730) Establishment Name Address ID/FEI Business Operations Yuki Gosei Kogyo Co., Ltd. 706298080 API MANUFACTURE(64842-1025, 64842-1020) Establishment Name Address ID/FEI Business Operations Taiho Pharmaceutical Co., Ltd. Saitama Plant 695734327 ANALYSIS(64842-1025, 64842-1020) , API MANUFACTURE(64842-1025, 64842-1020) Establishment Name Address ID/FEI Business Operations Taiho Pharmaceutical Co., Ltd. Kitajima Plant 692199778 MANUFACTURE(64842-1025, 64842-1020) , ANALYSIS(64842-1025, 64842-1020)
Trademark Results [LONSURF]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LONSURF 85742542 4438626 Live/Registered |
Taiho Pharmaceutical Co., Ltd. 2012-10-01 |
 LONSURF 79183501 5121586 Live/Registered |
TAIHO PHARMACEUTICAL CO., LTD. 2015-10-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.