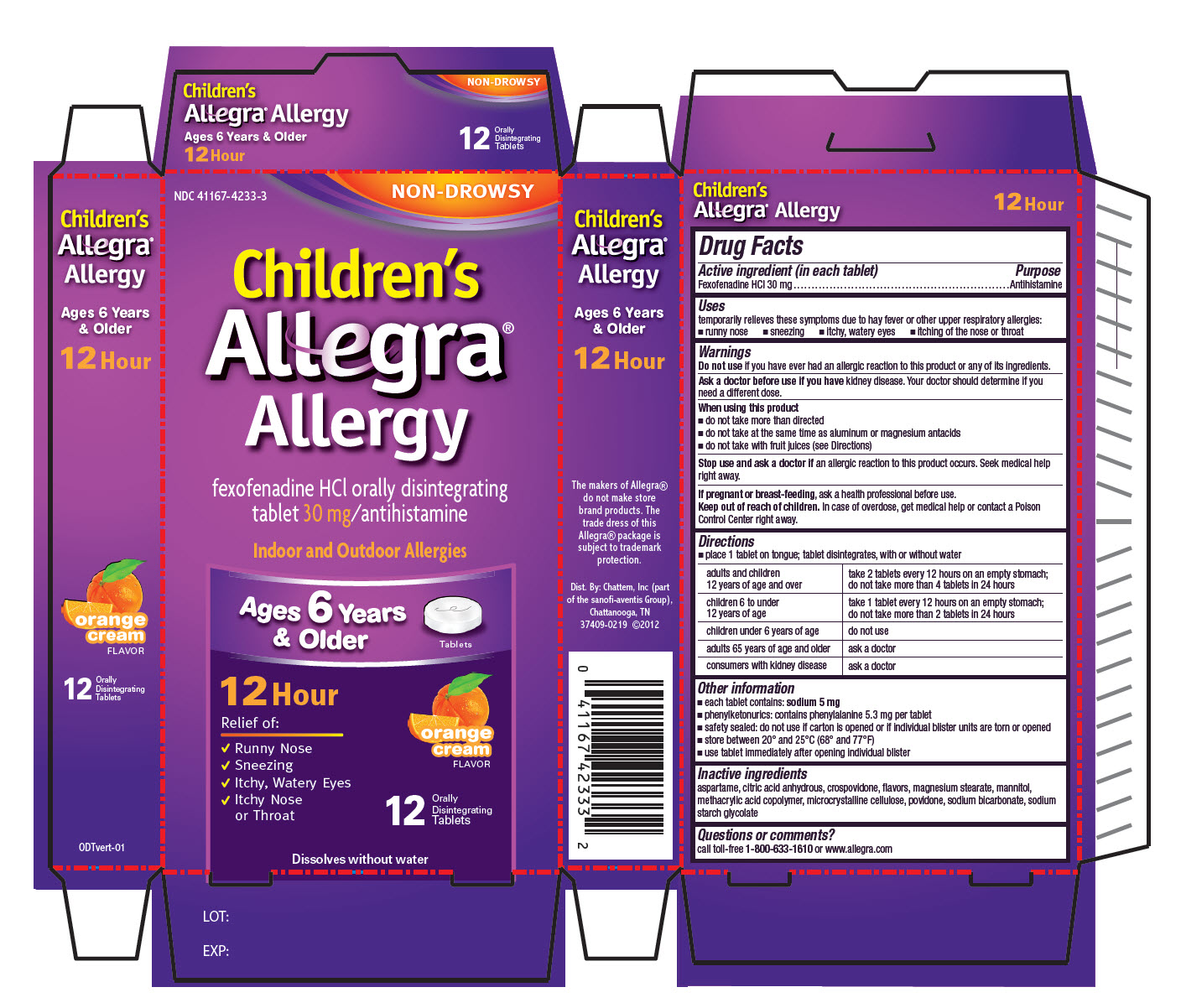
Childrens Allegra Allergy-ODT
Childrens Allegra Allergy by
Drug Labeling and Warnings
Childrens Allegra Allergy by is a Otc medication manufactured, distributed, or labeled by Chattem, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CHILDRENS ALLEGRA ALLERGY- fexofenadine hydrochloride tablet, orally disintegrating
Chattem, Inc.
----------
Childrens Allegra Allergy-ODT
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, water eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Directions
- place 1 tablet on tongue; tablet disintegrates, with or without water
| adults and children 12 years of age and over | take 2 tablets every 12 hours on an empty stomach; do not take more than 4 tablets in 24 hours |
| children 6 to under 12 years of age | take 1 tablet every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours |
| children under 6 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
- each tablet contains: sodium 5 mg
- phenylketonurics: contains phenylalanine 5.3 mg per tablet
- safety sealed: do not use if carton is opened or if individual blister units are torn or opened
- store between 20º and 25ºC (68º and 77ºF)
- use tablet immediately after opening individual blister
| CHILDRENS ALLEGRA ALLERGY
fexofenadine hydrochloride tablet, orally disintegrating |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Chattem, Inc. (003336013) |
Revised: 2/2019
Document Id: 30c89a70-49ab-46b2-b111-5bdea37848a4
Set id: f5eb861f-0ebd-4d37-a62a-5fecde4c65bd
Version: 13
Effective Time: 20190222
Chattem, Inc.