PROPOXYPHENE- propoxyphene hydrochloride capsule
Propoxyphene by
Drug Labeling and Warnings
Propoxyphene by is a Prescription medication manufactured, distributed, or labeled by Stat Rx USA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
DESCRIPTION
Propoxyphene hydrochloride, USP is an odorless, white crystalline powder with a bitter taste. It is freely soluble in water. Chemically, it is (2S,3R)-(+)-4-(Dimethylamino)-3-methyl-1,2-diphenyl-2-butanol propionate (ester) hydrochloride, which can be represented by the accompanying structural formula. Its molecular formula is C22H29NO2∙HCl and its molecular weight is 375.93.
Each capsule contains 65 mg (172.9 μmol) propoxyphene hydrochloride. It also contains the following inactive ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium lauryl sulfate. The empty hard-shell hypromellose capsule shells contain carnauba wax, carrageenan, hypromellose, potassium chloride, synthetic red iron oxide and titanium dioxide. In addition, the imprinting ink contains black iron oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.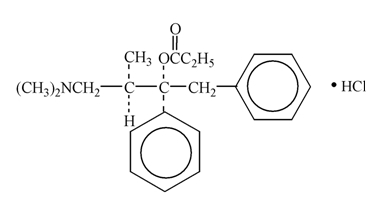
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGYPharmacology
Propoxyphene is a centrally acting opiate analgesic. In vitro studies demonstrated propoxyphene and the metabolite norpropoxyphene inhibit sodium channels (local anesthetic effect) with norpropoxyphene being approximately 2-fold more potent than propoxyphene and propoxyphene approximately 10-fold more potent than lidocaine. Propoxyphene and norpropoxyphene inhibit the voltage-gated potassium current carried by cardiac rapidly activating delayed rectifier (hERG) channels with approximately equal potency. It is unclear if the effects on ion channels occur within therapeutic dose range.
PharmacokineticsAbsorptionPeak plasma concentrations of propoxyphene are reached in 2 to 2.5 hours. After a 65 mg oral dose of propoxyphene hydrochloride, peak plasma levels of 0.05 to 0.1 mcg/mL for propoxyphene and 0.1 to 0.2 mcg /mL for norpropoxyphene (major metabolite) are achieved. Repeated doses of propoxyphene at 6 hour intervals lead to increasing plasma concentrations, with a plateau after the ninth dose at 48 hours. Propoxyphene has a half-life of 6 to 12 hours, whereas that of norpropoxyphene is 30 to 36 hours.
DistributionPropoxyphene is about 80% bound to proteins and has a large volume of distribution, 16 L/kg.
MetabolismPropoxyphene undergoes extensive first-pass metabolism by intestinal and hepatic enzymes. The major route of metabolism is cytochrome CYP3A4 mediated N-demethylation to norpropoxyphene, which is excreted by the kidneys. Ring hydroxylation and glucuronide formation are minor metabolic pathways.
ExcretionIn 48 hours, approximately 20% to 25% of the administered dose of propoxyphene is excreted via the urine, most of which is free or conjugated norpropoxyphene. The renal clearance rate of propoxyphene is 2.6 L/min.
Special PopulationsGeriatric PatientsAfter oral administration of propoxyphene in elderly patients (70 to 78 years), much longer half-lives of propoxyphene and norpropoxyphene have been reported (propropoxyphene 13 to 35 hours, norpropoxyphene 22 to 41 hours). In addition, the AUC was an average of 3-fold higher and the Cmax was an average of 2.5-fold higher in the elderly when compared to a younger (20 to 28 years) population. Longer dosage intervals may be considered in the elderly because the metabolism of propoxyphene may be reduced in this patient population. After multiple oral doses of propoxyphene in elderly patients (70 to 78 years), the Cmax of the metabolite (norpropoxyphene) was increased 5-fold.
Pediatric PatientsPropoxyphene has not been studied in pediatric patients.
Hepatic ImpairmentNo formal pharmacokinetic study of propoxyphene has been conducted in patients with mild, moderate or severe hepatic impairment.
After oral administration of propoxyphene in patients with cirrhosis, plasma concentrations of propoxyphene were considerably higher and norpropoxyphene concentrations were much lower than in control patients. This is presumably because of a decreased first-pass metabolism of orally administered propoxyphene in these patients. The AUC ratio of norpropoxyphene: propoxyphene was significantly lower in patients with cirrhosis (0.5 to 0.9) than in controls (2.5 to 4).
Renal ImpairmentNo formal pharmacokinetic study of propoxyphene has been conducted in patients with mild, moderate or severe renal impairment.
After oral administration of propoxyphene in anephric patients, the AUC and Cmax values were an average of 76% and 88% greater, respectively. Dialysis removes only insignificant amounts (8%) of administered dose of propoxyphene.
Drug InteractionsThe metabolism of propoxyphene may be altered by strong CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice and verapamil) leading to enhanced propoxyphene plasma levels. On the other hand, strong CYP3A4 inducers such as rifampin may lead to enhanced metabolite (norpropoxyphene) levels.
Propoxyphene is also thought to possess CYP3A4 and CYP2D6 enzyme inhibiting properties. Coadministration with a drug that is a substrate of CYP3A4 or CYP2D6, may result in higher plasma concentrations and increased pharmacologic or adverse effects of that drug.
- INDICATIONS & USAGE
-
CONTRAINDICATIONS
CONTRAINDICATIONS
Propoxyphene hydrochloride capsules are contraindicated in patients with known hypersensitivity to propoxyphene.
Propoxyphene hydrochloride capsules are contraindicated in patients with significant respiratory depression (in unmonitored settings or the absence of resuscitative equipment) and patients with acute or severe asthma or hypercarbia.
Propoxyphene hydrochloride capsules are contraindicated in any patient who has or is suspected of having paralytic ileus.
-
WARNINGS
WARNINGSRisk of Overdose
There have been numerous cases of accidental and intentional overdose with propoxyphene products either alone or in combination with other CNS depressants, including alcohol. Fatalities within the first hour of overdosage are not uncommon. Many of the propoxyphene-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation/attempts and/or concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants or other CNS-depressant drugs. Do not prescribe propoxyphene for patients who are suicidal or have a history of suicidal ideation.
Respiratory DepressionRespiratory depression is the chief hazard from all opioid agonist preparations. Respiratory depression occurs most frequently in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration. Propoxyphene should be used with extreme caution in patients with significant chronic obstructive pulmonary disease or cor pulmonale, and in patients having substantially decreased respiratory reserve, hypoxia, hypercapnia, or preexisting respiratory depression. In such patients, even usual therapeutic doses of propoxyphene may decrease respiratory drive to the point of apnea. In these patients alternative non-opioid analgesics should be considered and opioids should be employed only under careful medical supervision at the lowest effective dose.
Hypotensive EffectPropoxyphene, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines or other agents which compromise vasomotor tone. Propoxyphene may produce orthostatic hypotension in ambulatory patients. Propoxyphene, like all opioid analgesics, should be administered with caution to patients in circulatory shock, since vasodilatation produced by the drug may further reduce cardiac output and blood pressure.
Head Injury and Increased Intracranial PressureThe respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a preexisting increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Drug InteractionsThe concomitant use of propoxyphene and CNS depressants, including alcohol, can result in potentially serious adverse events including death. Because of its added depressant effects, propoxyphene should be prescribed with caution for those patients whose medical condition requires the concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants or other CNS-depressant drugs.
Usage in Ambulatory PatientsPropoxyphene may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
Use with AlcoholPatients should be cautioned about the concomitant use of propoxyphene products and alcohol because of potentially serious CNS-additive effects of these agents that can lead to death.
-
PRECAUTIONS
PRECAUTIONSTolerance and Physical Dependence
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Physical dependence and tolerance are not unusual during chronic opioid therapy.
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia and mydriasis. Other symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate or heart rate. In general, opioids should not be abruptly discontinued (see DOSAGE AND ADMINISTRATION: Cessation of Therapy).
If propoxyphene is abruptly discontinued in a physically dependent patient, an abstinence syndrome may occur (see DRUG ABUSE AND DEPENDENCE). If signs and symptoms of withdrawal occur, patients should be treated by reinstitution of opioid therapy followed by gradual tapered dose reduction of propoxyphene combined with symptomatic support (see DOSAGE AND ADMINISTRATION: Cessation of Therapy).
Use in Pancreatic/Biliary Tract DiseasePropoxyphene may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis. Opioids like propoxyphene may cause increases in the serum amylase level.
Hepatic or Renal ImpairmentInsufficient information exists to make appropriate dosing recommendations regarding the use of either propoxyphene in patients with hepatic or renal impairment as a function of degree of impairment. Higher plasma concentrations and/or delayed elimination may occur in case of impaired hepatic function and/or impaired renal function (see CLINICAL PHARMACOLOGY).
If the drug is used in these patients, it should be used with caution because of the hepatic metabolism and renal excretion of propoxyphene metabolites.
Information for Patients/Caregivers- Patients should be advised to report pain and adverse experiences occurring during therapy. Individualization of dosage is essential to make optimal use of this medication.
- Patients should be advised not to adjust the dose of propoxyphene without consulting the prescribing professional.
- Patients should be advised that propoxyphene may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating heavy machinery).
- Patients should not combine propoxyphene with central nervous system depressants (e.g., sleep aids, tranquilizers) except by the orders of the prescribing physician, because additive effects may occur.
- Patients should be instructed not to consume alcoholic beverages, including prescription and over-the-counter medications that contain alcohol, while using propoxyphene because of risk of serious adverse events including death.
- Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
- Patients should be advised that propoxyphene is a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
- Patients should be advised that if they have been receiving treatment with propoxyphene for more than a few weeks and cessation of therapy is indicated, it may be appropriate to taper the propoxyphene dose, rather than abruptly discontinue it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a gradual discontinuation of the medication.
Propoxyphene is metabolized mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4), therefore potential interactions may occur when propoxyphene is administered concurrently with agents that affect CYP3A4 activity.
The metabolism of propoxyphene may be altered by strong CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice and verapamil) leading to enhanced propoxyphene plasma levels. Coadministration with agents that induce CYP3A4 activity may reduce the efficacy of propoxyphene. Strong CYP3A4 inducers such as rifampin may lead to enhanced metabolite (norpropoxyphene) levels.
Propoxyphene is also thought to possess CYP3A4 and CYP2D6 enzyme inhibiting properties and coadministration with drugs that rely on either of these enzymes for metabolism may result in increased pharmacologic or adverse effects of that drug. Severe neurologic signs, including coma, have occurred with concurrent use of carbamazepine (metabolized by CYP3A4).
Increased risk of bleeding has been observed with warfarin-like agents when given along with propoxyphene; however, the mechanistic basis of this interaction is unknown.
CNS DepressantsPatients receiving narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with propoxyphene may exhibit an additive CNS depression. Interactive effects resulting in respiratory depression, hypotension, profound sedation or coma may result if these drugs are taken in combination with the usual dosage of propoxyphene. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Mixed Agonist/Antagonist Opioid AnalgesicsAgonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol and buprenorphine) should be administered with caution to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic such as propoxyphene. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of propoxyphene and/or may precipitate withdrawal symptoms in these patients.
Monoamine Oxidase Inhibitors (MAOIs)MAOIs have been reported to intensify the effects of at least one opioid drug causing anxiety, confusion and significant depression of respiration or coma. The use of propoxyphene is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
Carcinogenesis, Mutagenesis, Impairment of FertilityThe mutagenic and carcinogenic potential of propoxyphene has not been evaluated.
In animal studies there was no effect of propoxyphene on mating behavior, fertility, duration of gestation or parturition when rats were fed propoxyphene as a component of their daily diet at estimated daily propoxyphene intake up to 8-fold greater than the maximum human equivalent dose (HED) based on body surface area comparison. At this highest dose, fetal weight and survival on postnatal day 4 was reduced
PregnancyRisk SummaryPregnancy Category CThere are no adequate and well controlled studies of propoxyphene in pregnant women. While there are limited data in the published literature, adequate animal reproduction studies have not been conducted with propoxyphene. Therefore, it is not known whether propoxyphene can affect reproduction or cause fetal harm when administered to a pregnant woman. Propoxyphene should be given to a pregnant woman only if clearly needed.
Clinical ConsiderationsPropoxyphene and its major metabolite, norpropoxyphene, cross the human placenta. Neonates whose mothers have taken opiates chronically may exhibit respiratory depression or withdrawal symptoms.
DataIn published animal reproduction studies, no teratogenic effects occurred in offspring born to pregnant rats or rabbits that received propoxyphene during organogenesis. Pregnant animals received propoxyphene doses approximately 10-fold (rats) and 4-fold (rabbits) the maximum recommended human dose (based on mg/m2 body surface area comparison).
Nursing MothersPropoxyphene, norpropoxyphene (major metabolite), are excreted in human milk. Published studies of nursing mothers using propoxyphene detected no adverse effects in nursing infants. Based on a study of six mother-infant pairs, an exclusively breastfed infant receives approximately 2% of the maternal weight-adjusted dose. Norpropoxyphene is renally excreted and renal clearance is lower in neonates than in adults. Therefore, it is possible that prolonged maternal propoxyphene use could result in norpropoxyphene accumulation in a breastfed infant. Watch breast-feeding infants for signs of sedation including poor feeding, somnolence or respiratory depression. Caution should be exercised when propoxyphene is administered to a nursing woman.
Pediatric UseSafety and effectiveness in pediatric patients have not been established.
Elderly PatientsClinical studies of propoxyphene did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, post-marketing reports suggest that patients over the age of 65 may be more susceptible to CNS-related side effects. Therefore, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. Decreased total daily dosage should be considered (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
ADVERSE REACTIONS
In hospitalized patients, the most frequently reported were dizziness, sedation, nausea and vomiting. Other adverse reactions include constipation, abdominal pain, skin rashes, lightheadedness, headache, weakness, euphoria, dysphoria, hallucinations and minor visual disturbances.
The most frequently reported post-marketing adverse events have included completed suicide, accidental and intentional overdose, drug dependence, cardiac arrest, coma, drug ineffective, drug toxicity, nausea, respiratory arrest, cardio-respiratory arrest, death, vomiting, dizziness, convulsion, confusional state and diarrhea.
Additional adverse experiences reported through post-marketing surveillance include:
Cardiac Disorders: arrhythmia, bradycardia, cardiac/respiratory arrest, congestive arrest, congestive heart failure (CHF), tachycardia, myocardial infarction (MI)
Eye Disorder: eye swelling, vision blurred
General Disorder and Administration Site Conditions: drug interaction, drug tolerance, drug withdrawal syndrome
Gastrointestinal Disorder: gastrointestinal bleed, acute pancreatitis
Hepatobiliary Disorder: hepatic steatosis, hepatomegaly, hepatocellular injury
Immune System Disorder: hypersensitivity
Injury Poisoning and Procedural Complications: drug toxicity, hip fracture, multiple drug overdose, narcotic overdose
Investigations: blood pressure decreased, heart rate elevated/abnormal
Metabolism and Nutrition Disorder: metabolic acidosis
Nervous System Disorder: ataxia, coma, dizziness, somnolence, syncope
Psychiatric: abnormal behavior, confusional state, hallucinations, mental status change
Respiratory, Thoracic and Mediastinal Disorders: respiratory depression, dyspnoea
Skin and Subcutaneous Tissue Disorder: rash, itch.
Liver dysfunction has been reported in association with propoxyphene. Propoxyphene therapy has been associated with abnormal liver function tests and, more rarely, with instances of reversible jaundice (including cholestatic jaundice).
Subacute painful myopathy has been reported following chronic propoxyphene overdosage.
-
OVERDOSAGE
OVERDOSAGE
Overdose of propoxyphene may present with the signs and symptoms of propoxyphene overdose. Fatalities within the first hour of overdosage are not uncommon.
In all cases of suspected overdosage, call your regional Poison Control Center to obtain the most up-to-date information about the treatment of overdose. This recommendation is made because, in general, information regarding the treatment of overdosage may change more rapidly than do package inserts.
Initial consideration should be given to the management of the CNS effects of propoxyphene overdosage. Resuscitative measures should be initiated promptly.
Symptoms of Propoxyphene OverdosageThe manifestations of acute overdosage with propoxyphene are those of narcotic overdosage. The patient is usually somnolent but may be stuporous or comatose and convulsing. Respiratory depression is characteristic. The ventilatory rate and/or tidal volume is decreased, which results in cyanosis and hypoxia. Pupils, initially pinpoint, may become dilated as hypoxia increases. Cheyne-Stokes respiration and apnea may occur. Blood pressure and heart rate are usually normal initially, but blood pressure falls and cardiac performance deteriorates, which ultimately results in pulmonary edema and circulatory collapse, unless the respiratory depression is corrected and adequate ventilation is restored promptly. Cardiac arrhythmias and conduction delay may be present. A combined respiratory-metabolic acidosis occurs owing to retained CO2 (hypercapnia) and to lactic acid formed during anaerobic glycolysis. Acidosis may be severe if large amounts of salicylates have also been ingested. Death may occur.
Treatment of Propoxyphene OverdosageAttention should be directed first to establishing a patent airway and to restoring ventilation. Mechanically assisted ventilation, with or without oxygen, may be required, and positive pressure respiration may be desirable if pulmonary edema is present. The opioid antagonist naloxone will markedly reduce the degree of respiratory depression, and should be administered promptly, preferably intravenously. The duration of action of the antagonist may be brief. If no response is observed after 10 mg of naloxone have been administered, the diagnosis of propoxyphene toxicity should be questioned.
In addition to the use of an opioid antagonist, the patient may require careful titration with an anticonvulsant to control convulsions. Activated charcoal can adsorb a significant amount of ingested propoxyphene. Dialysis is of little value in poisoning due to propoxyphene. Efforts should be made to determine whether other agents, such as alcohol, barbiturates, tranquilizers or other CNS depressants, were also ingested, since these increase CNS depression as well as cause specific toxic effects or death.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Propoxyphene hydrochloride capsules are intended for the management of mild to moderate pain. The dose should be individually adjusted according to severity of pain, patient response and patient size.
Propoxyphene hydrochloride capsules are given orally. The usual dosage is one 65 mg propoxyphene hydrochloride capsule every 4 hours as needed for pain. The maximum dose of propoxyphene hydrochloride capsules is 6 capsules per day. Do not exceed the maximum daily dose.
Patients receiving propoxyphene and any CYP3A4 inhibitor should be carefully monitored for an extended period of time and dosage adjustments should be made if warranted.
Consideration should be given to a reduced total daily dosage in elderly patients and in patients with hepatic or renal impairment.
Cessation of TherapyFor patients who used propoxyphene hydrochloride capsules on a regular basis for a period of time, when therapy with propoxyphene hydrochloride capsules is no longer needed for the treatment of their pain, it may be useful to gradually discontinue the propoxyphene hydrochloride capsules over time to prevent the development of an opioid abstinence syndrome (narcotic withdrawal). In general, therapy can be decreased by 25% to 50% per day with careful monitoring for signs and symptoms of withdrawal (see DRUG ABUSE AND DEPENDENCE for description of the signs and symptoms of withdrawal). If the patient develops these signs or symptoms, the dose should be raised to the previous level and titrated down more slowly, either by increasing the interval between decreases, decreasing the amount of change in dose, or both.
-
HOW SUPPLIED
HOW SUPPLIED
Propoxyphene Hydrochloride Capsules, USP are available containing 65 mg of propoxyphene hydrochloride, USP.
The 65 mg capsule is a hard-shell hypromellose capsule with a rose opaque cap and a rose opaque body, axially printed with MYLAN over 7065 in black ink on both the cap and the body. The capsule is filled with white to off-white powder. They are available as follows:
NDC: 0378-7065-01
bottles of 100 capsulesNDC: 0378-7065-05
bottles of 500 capsulesStore at 20º to 25ºC (68º to 77ºF). [See USP Controlled Room Temperature.]
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
-
BOXED WARNING
(What is this?)
WARNINGS
- There have been numerous cases of accidental and intentional overdose with propoxyphene products either alone or in combination with other CNS depressants, including alcohol. Fatalities within the first hour of overdosage are not uncommon. Many of the propoxyphene-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation/attempts and/or concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants or other CNS-depressant drugs. Do not prescribe propoxyphene for patients who are suicidal or have a history of suicidal ideation.
- The metabolism of propoxyphene may be altered by strong CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice and verapamil) leading to enhanced propoxyphene plasma levels. Patients receiving propoxyphene and any CYP3A4 inhibitor should be carefully monitored for an extended period of time and dosage adjustments should be made if warranted (see CLINICAL PHARMACOLOGY: Drug Interactions and WARNINGS, PRECAUTIONS and DOSAGE AND ADMINISTRATION for further information).
-
INFORMATION FOR PATIENTS
PROPOXYPHENE HYDROCHLORIDE - propoxyphene hydrochloride capsule
Mylan Pharmaceuticals Inc.
----------
MEDICATION GUIDE
PROPOXYPHENE HYDROCHLORIDE CAPSULES, USPRead this Medication Guide before you start taking propoxyphene hydrochloride capsules, and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is the most important information I should know about propoxyphene hydrochloride capsules?
Propoxyphene hydrochloride capsules and other medicines that contain propoxyphene can cause serious side effects, including:
Overdoses by accident or on purpose (intentional overdose). Overdoses with propoxyphene hydrochloride capsules may happen when it is taken by itself, or with alcohol or other medicines that can also decrease your breathing and make you very sleepy.
-
Death can happen within 1 hour of taking an overdose of
propoxyphene hydrochloride capsules.
Many of the deaths that happen in people who take propoxyphene hydrochloride capsules happen in those who:
- have emotional problems
- have thoughts of suicide or attempted suicide, or
- also take antidepressants, sedatives, tranquilizers, muscle relaxants or other medicines that affect your breathing and make you very sleepy. You should not use any of these medicines with propoxyphene hydrochloride capsules without talking to your doctor.
-
Before taking propoxyphene hydrochloride capsules tell your
doctor if you:
- have a lung problem, such as COPD or cor pulmonale
- have liver or kidney problems
- have problems with your pancreas or gallbladder
- have a history of head injury
- are over age 65
- have a history of drug or alcohol abuse or addiction
Take propoxyphene hydrochloride capsules exactly as prescribed. Do not change your dose or stop taking propoxyphene hydrochloride capsules without first talking to your doctor.
- If you take propoxyphene hydrochloride capsules, do not take more than 6 capsules in one day.
- Before taking propoxyphene hydrochloride capsules, tell your doctor about all the medicines you take. Propoxyphene hydrochloride capsules and many other medicines may interact with each other and may cause serious side effects. Certain medicines can affect how your liver breaks down other medicines. See “What should I tell my doctor before taking propoxyphene hydrochloride capsules?”
- Do not drink grapefruit juice or eat grapefruit while you take propoxyphene hydrochloride capsules. Grapefruit juice may interact with propoxyphene hydrochloride capsules.
- Do not drink alcohol while using propoxyphene hydrochloride capsules. Using alcohol with propoxyphene hydrochloride capsules may increase your risk of having dangerous side effects.
What are propoxyphene hydrochloride capsules?
- Propoxyphene hydrochloride capsules are a prescription medicine used to treat mild to moderate pain.
- Propoxyphene hydrochloride capsules are a federally controlled substance (C-IV) because they are strong opioid pain medicines that can be abused by people who abuse prescription medicines or street drugs.
- Prevent theft, misuse or abuse. Keep propoxyphene hydrochloride capsules in a safe place to protect it from being stolen. Propoxyphene hydrochloride capsules can be a target for people who misuse or abuse prescription medicines or street drugs.
- Never give propoxyphene hydrochloride capsules to anyone else, even if they have the same symptoms that you have. It may harm them or even cause death. Selling or giving away this medicine is against the law.
It is not known if propoxyphene hydrochloride capsules are safe and effective in children younger than age 18.
Who should not take propoxyphene hydrochloride capsules?
Do not take propoxyphene hydrochloride capsules if you:
- are allergic to propoxyphene. Ask your doctor if you are not sure. See the end of this Medication Guide for a list of the ingredients in propoxyphene hydrochloride capsules.
- are having an asthma attack or have severe asthma, trouble breathing or have a lung problem
- have a bowel blockage called paralytic ileus
What should I tell my doctor before taking propoxyphene hydrochloride capsules?
Before you take propoxyphene hydrochloride capsules, tell your doctor:
- if you have any of the conditions listed in the section “What is the most important information I should know about propoxyphene hydrochloride capsules?”
- if you are allergic to propoxyphene
- if you plan to have surgery with general anesthesia
- if you are pregnant or plan to become pregnant.
- if you take propoxyphene hydrochloride capsules regularly before your baby
is born, your newborn baby may have withdrawal symptoms because their body has
become used to the medicine. Symptoms of withdrawal in a newborn baby may
include:
- irritability
- shaking (tremors)
- jitteriness
- breathing faster than normal
- crying more than usual
- diarrhea or more stools than normal
- vomiting
- fever
- if you take propoxyphene hydrochloride capsules right before your baby is born, your baby could have breathing problems.
- if you are breast-feeding or plan to breast-feed. Some propoxyphene
hydrochloride capsules passes into breast milk.
Tell your doctor about all the medicines you take, including prescription, and nonprescription medicines, vitamins and herbal supplements. Propoxyphene hydrochloride capsules interact with many medicines and may lead to serious side effects. The doses of certain medicines may need to be changed.
Especially tell your doctor if you take:
See “What is the most important information I should know about propoxyphene hydrochloride capsules?”
- certain medicines that can affect how your liver breaks down other medicines
- a monoamine oxidase inhibitor (MAOI) medicine
- other medicines that make you sleepy, such as: other medicines for pain, including other opioid medicines, antidepressant medicines, sleeping pills, anti-anxiety medicines, muscle relaxants, anti-nausea medicines or tranquilizers
- a blood pressure medicine
- a blood-thinner medicine. You may have an increased risk of bleeding while also taking propoxyphene hydrochloride capsules.
Ask your doctor or pharmacist if you are not sure if your medicine is one listed above.
Know the medicines you take. Keep a list of them to show to your doctor and pharmacist when you get a new medicine.
How should I take propoxyphene hydrochloride capsules?
See “What is the most important information I should know about propoxyphene hydrochloride capsules?”
- Take propoxyphene hydrochloride capsules exactly as prescribed.
- If you take too much propoxyphene hydrochloride capsules, or take it with alcohol or other medicines, you may overdose. See “What is the most important information I should know about propoxyphene hydrochloride capsules?” You will need medical help right away if you think you have taken an overdose of propoxyphene hydrochloride capsules. A large overdose could cause you to become unconscious and die.
Signs and symptoms of an overdose of propoxyphene hydrochloride capsules include:
- you are very sleepy or do not respond to others
- confusion
- have trouble breathing or stop breathing
- changes in blood pressure and heart rate
What are the possible side effects of propoxyphene hydrochloride capsules?
Propoxyphene hydrochloride capsules can cause serious side effects, including:
See “What is the most important information I should know about propoxyphene hydrochloride capsules?”
-
Serious breathing problems that can become life
threatening. This is especially true if you already have a serious lung
or breathing problem, or your body is not used to opioid pain medicines. This
can happen even if you take propoxyphene hydrochloride capsules exactly as
prescribed by your doctor. Call your doctor or get medical help right away if:
- your breathing slows down
- you have shallow breathing (little chest movement with breathing)
- you feel faint, dizzy, confused, or
- you have any other unusual symptoms
- Propoxyphene hydrochloride capsules can cause your blood pressure to drop. This can make you feel dizzy and faint if you get up too fast from sitting or lying down. Low blood pressure is also more likely to happen if you take other medicines that can also lower your blood pressure. Severe low blood pressure can happen if you lose blood or take certain other medicines.
- Sleepiness. Propoxyphene hydrochloride capsules can cause sleepiness and may affect your ability to make decisions, think clearly, or react quickly. Do not drive, operate heavy machinery, or do other dangerous activities until you know how propoxyphene hydrochloride capsules affects you.
-
Propoxyphene hydrochloride capsules can cause physical
dependence if you take it for more than a few weeks. Do not stop taking
propoxyphene hydrochloride capsules all of a sudden. You could become sick with
uncomfortable withdrawal symptoms (for example, nausea, vomiting, diarrhea,
anxiety and shivering) because your body has become used to the medicine.
Physical dependence is not the same as drug addiction. Your doctor can tell you
more about the differences between physical dependence and drug addiction.
Tell your doctor if you have any of these withdrawal symptoms while you slowly stop taking propoxyphene hydrochloride capsules. You may need to stop propoxyphene hydrochloride capsules more slowly.
Common side effects of propoxyphene hydrochloride capsules include:
- dizziness
- feeling sleepy
- nausea and vomiting
- constipation
- stomach area (abdominal) pain
- skin rashes
- lightheadedness
- headache
- weakness
- feeling of excitement (elation) or discomfort
- seeing, hearing or sensing things that are not really there (hallucinations)
- blurred vision
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Mylan Pharmaceuticals, Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
How should I store propoxyphene hydrochloride capsules?
- Store propoxyphene hydrochloride capsules at 20oC to 25oC (68oF to 77oF).
Keep propoxyphene hydrochloride capsules and all medicines out of the reach of children.
General information about propoxyphene hydrochloride capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use propoxyphene hydrochloride capsules for a purpose for which it was not prescribed. Do not give propoxyphene hydrochloride capsules to others even if they have the same symptoms you have. It may harm them and is against the law.
This Medication Guide summarizes the most important information about propoxyphene hydrochloride capsules. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about propoxyphene hydrochloride capsules that is written for health professionals. For more information, call Mylan Pharmaceuticals, Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
What are the ingredients in propoxyphene hydrochloride capsules, USP?
Active ingredient: propoxyphene hydrochloride, USP
Inactive ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium lauryl sulfate. The empty hard-shell hypromellose capsule shells contain carnauba wax, carrageenan, hypromellose, potassium chloride, synthetic red iron oxide and titanium dioxide. In addition, the imprinting ink contains black iron oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505OCTOBER 2009
Revised: 10/2009Mylan Pharmaceuticals Inc.
MG:PHCL:R1 -
Death can happen within 1 hour of taking an overdose of
propoxyphene hydrochloride capsules.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROPOXYPHENE
propoxyphene hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16590-198(NDC: 0378-7065) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPOXYPHENE HYDROCHLORIDE (UNII: CB2TL9PS0T) (PROPOXYPHENE - UNII:S2F83W92TK) PROPOXYPHENE HYDROCHLORIDE 65 mg Product Characteristics Color pink Score no score Shape CAPSULE Size 16mm Flavor Imprint Code MYLAN;7065 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16590-198-30 30 in 1 BOTTLE 2 NDC: 16590-198-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040569 10/27/2009 Labeler - Stat Rx USA (786036330)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
