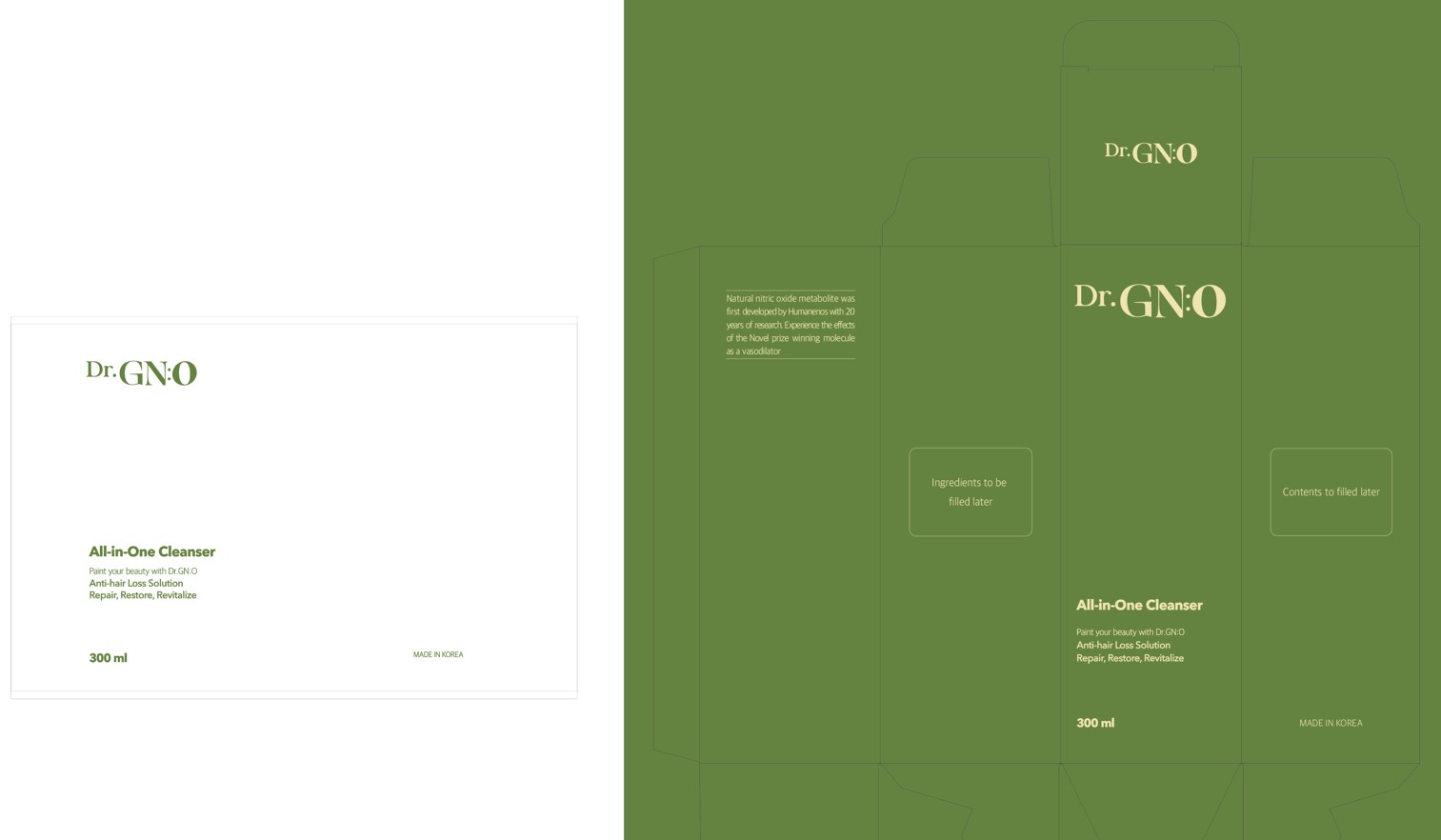
Dr. GNO Cleansing Soap (Helps cleanse sensitive skin, clears skin troubles, especially acne, Fermented Cabbage Extract, Mesoporous Silica Nanoparticle, Zinc)
Dr. GNO CLEANSER (Helps cleanse sensitive skin, clears skin troubles, especially acne, fermented cabbage extract, mesoporous silica nanoparticle, zinc) by
Drug Labeling and Warnings
Dr. GNO CLEANSER (Helps cleanse sensitive skin, clears skin troubles, especially acne, fermented cabbage extract, mesoporous silica nanoparticle, zinc) by is a Otc medication manufactured, distributed, or labeled by HumanEnos LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DR. GNO CLEANSER (HELPS CLEANSE SENSITIVE SKIN, CLEARS SKIN TROUBLES, ESPECIALLY ACNE, FERMENTED CABBAGE EXTRACT, MESOPOROUS SILICA NANOPARTICLE, ZINC)- nitric oxide, zinc soap
HumanEnos LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Dr. GNO Cleansing Soap (Helps cleanse sensitive skin, clears skin troubles, especially acne, Fermented Cabbage Extract, Mesoporous Silica Nanoparticle, Zinc)
| DR. GNO CLEANSER (HELPS CLEANSE SENSITIVE SKIN, CLEARS SKIN TROUBLES, ESPECIALLY ACNE, FERMENTED CABBAGE EXTRACT, MESOPOROUS SILICA NANOPARTICLE, ZINC)
nitric oxide, zinc soap |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - HumanEnos LLC (695801540) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HumanEnos LLC | 695801540 | manufacture(83202-2139) | |
Revised: 3/2023
Document Id: f6197c69-28b7-150e-e053-2995a90a7645
Set id: f6197c69-28b6-150e-e053-2995a90a7645
Version: 1
Effective Time: 20230304
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.