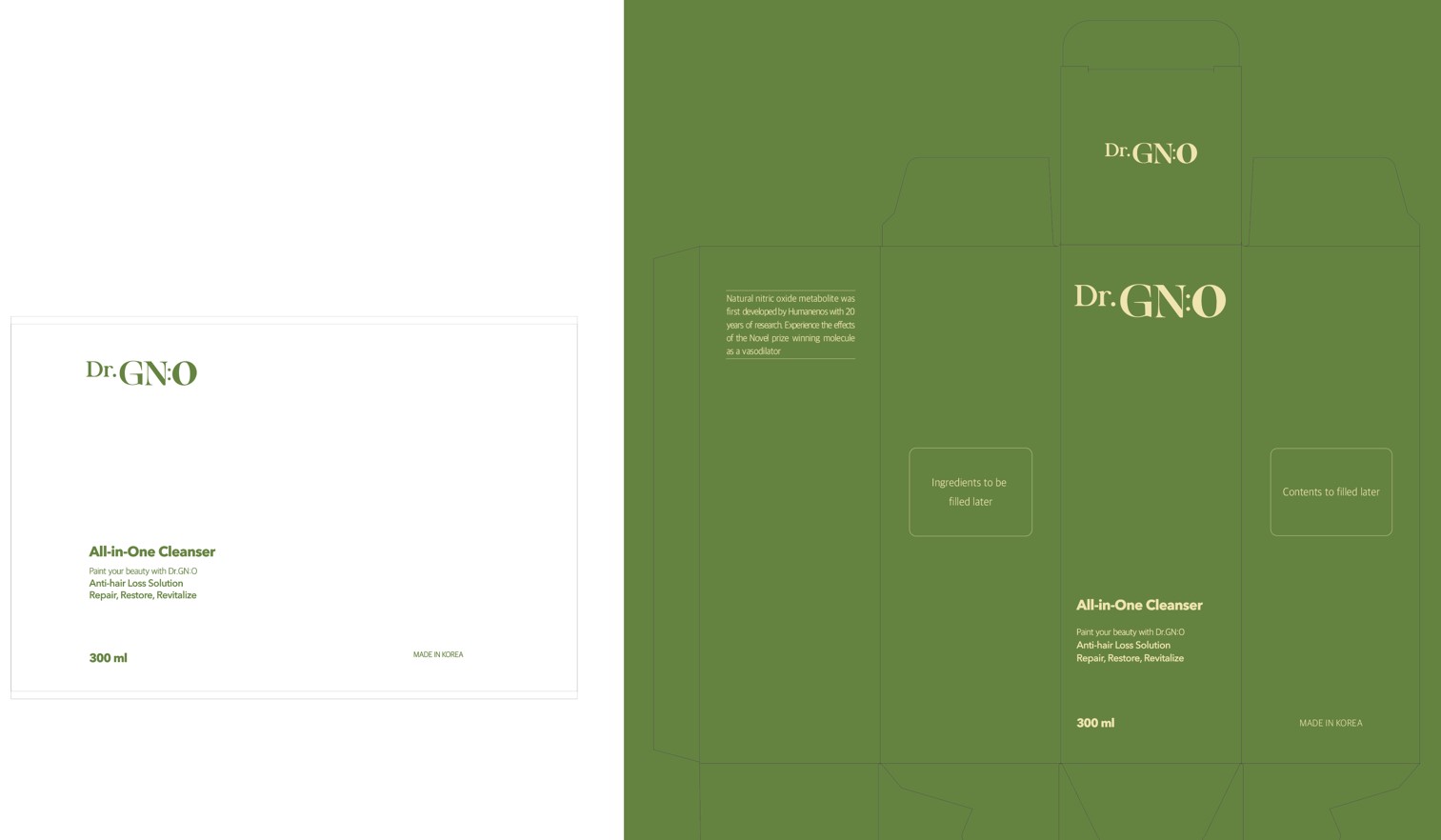
DR. GNO ALL-IN-ONE CLEANSER (PREVENTS HAIRLOSS, FERMENTED LETTUCE, GARLIC, BEAN, CABBAGE, CRICKET GREENTEA LEAF, MULBERRY BRANCH, LEAF, AND ROOTS EXTRACT MESOPOROUS SILICA NANOPARTICLE, ZINC) NITRIC OXIDE, DEXPANTHENOL, NIACINAMIDE, SALICYLIC ACID, ZINC- nitric oxide, dexpanthenol, niacinamide, salicylic acid, zinc liquid
HumanEnos LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Dr. GNO All-in-One Cleanser (Prevents hairloss, Fermented lettuce, garlic, bean, cabbage, cricket Greentea leaf, Mulberry branch, leaf, and roots extract Mesoporous Silica Nanoparticle, Zinc)
Dr.GN:O All-in-One Cleanser
KEEP OUT OF THE REACH OF THE CHILDREN.
Dr.GN:O All-in-One Cleanser
Dr.GN:O All-in-One Cleanser
Inactive ingredients:
Water, Glycerin, Lavender oil
Active ingredients:
Nitric Oxide, Dexpanthenol, Niacinamide, Salicylic acid, Zinc
Dr.GN:O All-in-One Cleanser
DR. GNO ALL-IN-ONE CLEANSER (PREVENTS HAIRLOSS, FERMENTED LETTUCE, GARLIC, BEAN, CABBAGE, CRICKET GREENTEA LEAF, MULBERRY BRANCH, LEAF, AND ROOTS EXTRACT MESOPOROUS SILICA NANOPARTICLE, ZINC)
NITRIC OXIDE, DEXPANTHENOL, NIACINAMIDE, SALICYLIC ACID, ZINC
nitric oxide, dexpanthenol, niacinamide, salicylic acid, zinc liquid |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 83202-6712 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| NITRIC OXIDE (UNII: 31C4KY9ESH) (NITRIC OXIDE - UNII:31C4KY9ESH) | NITRIC OXIDE | 100 mg in 300 mg |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| WATER (UNII: 059QF0KO0R) | 1 mg in 300 mg |
|
| Product Characteristics |
| Color | white | Score | score with uneven pieces |
| Shape | CAPSULE | Size | 300mm |
| Flavor | FRUIT | Imprint Code |
liquid
|
| Contains | |
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 83202-6712-3 | 300 mg in 1 PACKAGE; Type 0: Not a Combination Product | 02/14/2023 | 02/13/2024 |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | | 02/14/2023 | 02/13/2024 |
|
| Labeler - HumanEnos LLC
(695801540)
|
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| HumanEnos LLC | | 695801540 | manufacture(83202-6712) |