GONAK HYPROMELLOSE (hypromellose 2906 (4000 mpa.s) and hypromellose 2906- 50 mpa.s solution
Gonak Hypromellose by
Drug Labeling and Warnings
Gonak Hypromellose by is a Prescription medication manufactured, distributed, or labeled by Akorn, Akorn Operating Company LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
WARNINGS:
To Avoid contamination, do not touch tip of container to any surface.
Replace cap after using.
Not for use in conjunction with hot laser treatment. If solution changes color or becomes cloudy, do not use.DO NOT USE IF IMPRINTED SEAL ON BOTTLE NECK IS BROKEN OR MISSING
Each mL contains:
Active: Hypromellose 52906, 25mg (2.5%).Inactives: Boric Acid, Edetate Disodium, Potassium Chloride, Sodium Borate, Purified Water, Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (6.0 to 7.8).
Preservative: Benzalkonium Chloride 0.01%.
KEEP OUT OF REACH OF CHILDREN
-
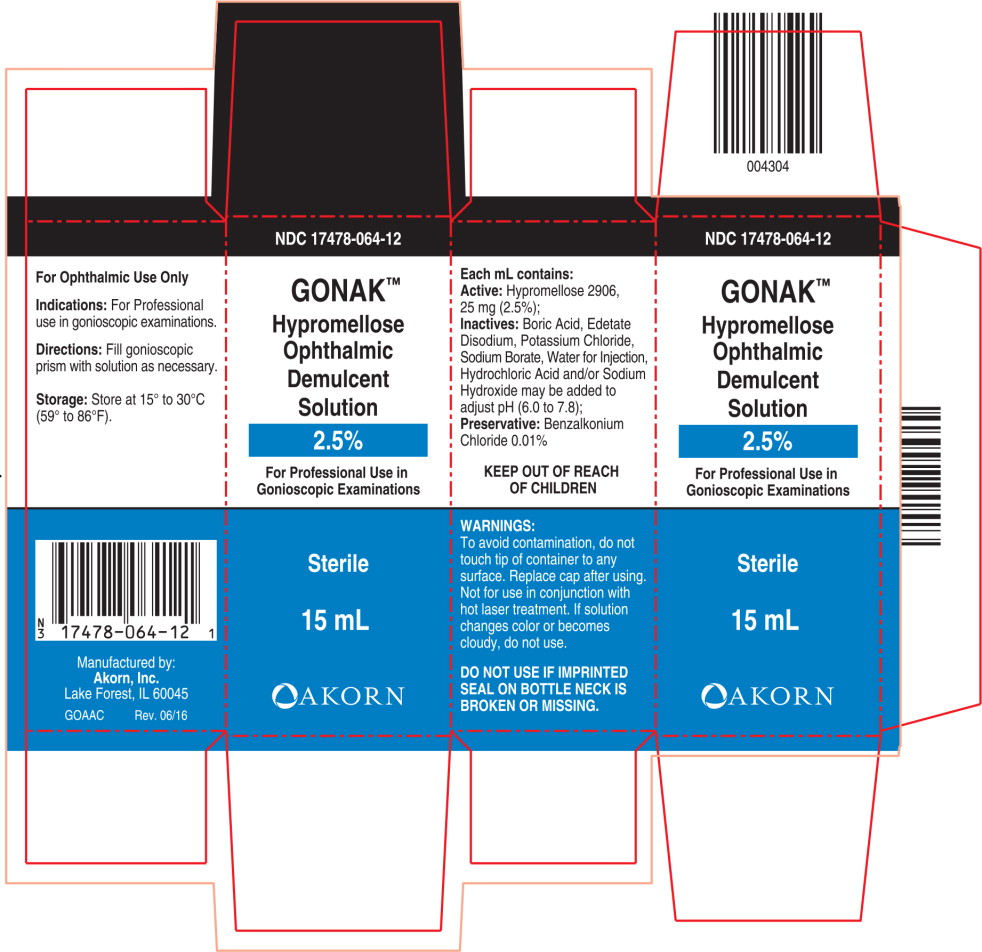
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 17478-064-12
Gonak™
Hypromellose
Ophthalmic
Demulcent
Solution
2.5%
For Professional Use In
Gonioscopic Examinations
FOR OPTHALMIC USE ONLY
15 mL Sterile
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-064-12
Gonak™
Hypromellose
Ophthalmic
Demulcent
Solution
2.5%
For Professional Use In
Gonioscopic Examinations
15 mL
Sterile
[Akorn Logo]
-
INGREDIENTS AND APPEARANCE
GONAK HYPROMELLOSE
hypromellose 2906 (4000 mpa.s) and hypromellose 2906 (50 mpa.s) solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-064 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hypromellose 2906 (4000 MPA.S) (UNII: 5EYA69XGAT) (Hypromellose 2906 (4000 MPA.S) - UNII:5EYA69XGAT) Hypromellose 2906 (4000 MPA.S) 25 mg in 1 mL Hypromellose 2906 (50 MPA.S) (UNII: 612E703ZUQ) (Hypromellose 2906 (50 MPA.S) - UNII:612E703ZUQ) Hypromellose 2906 (50 MPA.S) 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength boric acid (UNII: R57ZHV85D4) edetate disodium (UNII: 7FLD91C86K) potassium chloride (UNII: 660YQ98I10) sodium borate (UNII: 91MBZ8H3QO) water (UNII: 059QF0KO0R) sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-064-12 1 in 1 CARTON 01/01/1997 1 15 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 01/01/1997 Labeler - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 603980319 MANUFACTURE(17478-064) , REPACK(17478-064) , ANALYSIS(17478-064)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.