Holus Relief Gel, 1.7 oz by Nemadji Management, LLC HOLUS RELIEF GEL, 1.7 OZ
Holus Relief Gel, 1.7 oz by
Drug Labeling and Warnings
Holus Relief Gel, 1.7 oz by is a Otc medication manufactured, distributed, or labeled by Nemadji Management, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
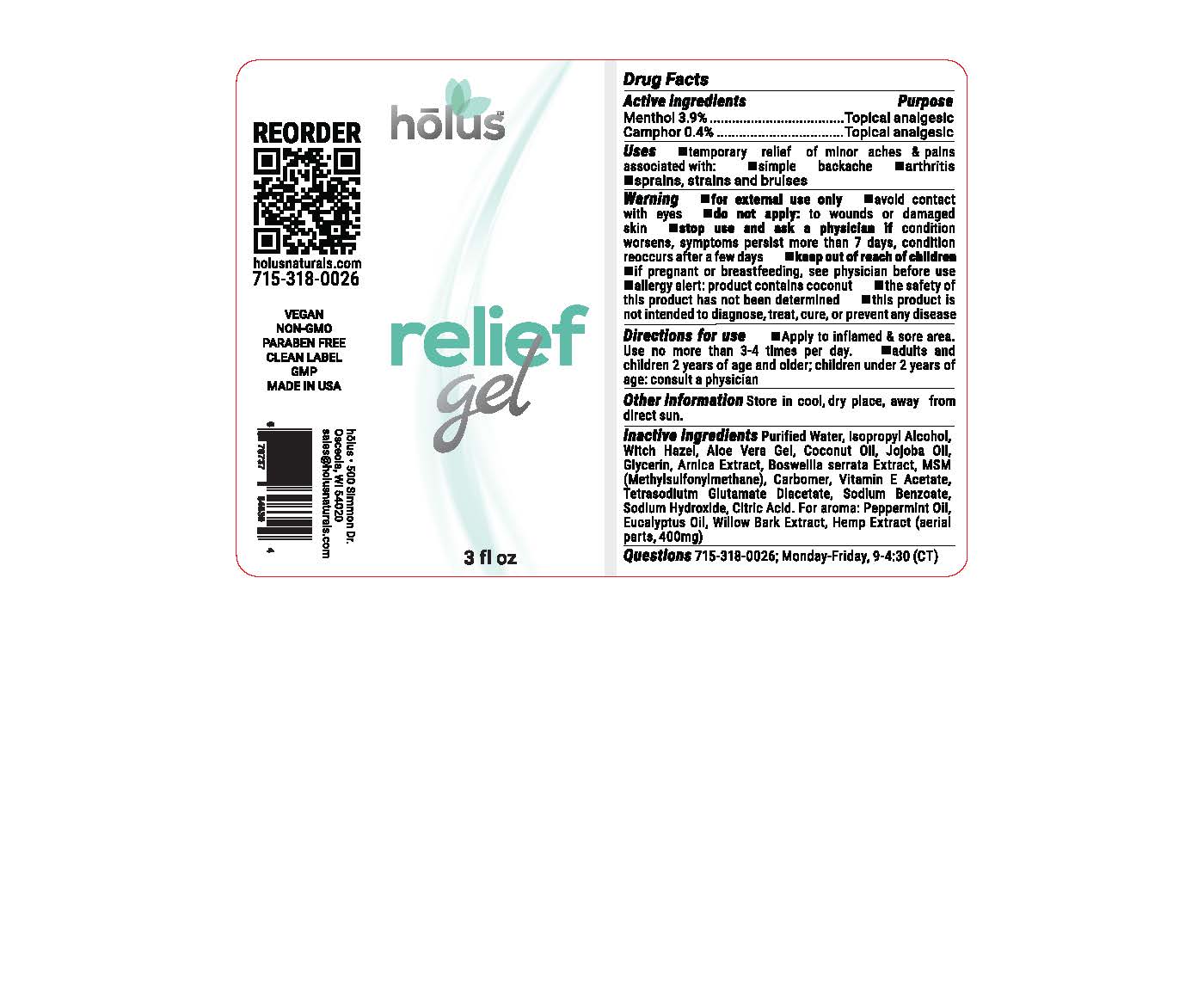
HOLUS RELIEF GEL, 1.7 OZ- menthol, camphor gel
Nemadji Management, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
HOLUS RELIEF GEL, 1.7 OZ
DESCRIPTION
Holus Relief Gel, 1.7oz - menthol, camphor gel
Topical analgesic, Over-The-Counter drug
1.7oz ounces of topical analgesic gel in a pump dispenser
Disclaimer: Most OTC drugs are not reviewed and approved by the FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
PURPOSE
FOR THE TEMPORARY RELIEF OF MINOR ACHES AND PAINS OF MUSCLES AND JOINTS ASSOCIATED WITH SIMPLE BACKACHE, ARTHRITIS, STRAINS, BRUISES AND SPRAINS.
WARNINGS
FOR EXTERNAL USE ONLY
AVOID CONTACT WITH EYES
DO NOT APPLY: TO WOUNDS OR DAMAGED SKIN
ALLERGY: PRODUCT CONTAINS COCONUT
THE SAFETY OF THIS PRODUCT HAS NOT BEEN DETERMINED.
THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE, OR PREVENT ANY DISEASE
INACTIVE INGREDIENTS, Holus Relief Gel (1.7oz)
PURIFIED WATER, ISOPROPYL ALCOHOL, WITCH HAZEL, ALOE VERA GEL, COCONUT OIL, JOJOBA OIL, GLYCERIN, ARNICA EXTRACT, BOSWELLIA SERRATA EXTRACT, MSM (METHYLSULFONYLMETHANE), CARBOMER, VITAMIN E ACETATE, TETRASODIUM GLUTAMATE DIACETATE, SODIUM BENZOATE, SODIUM HYDROXIDE, CITRIC ACID, PEPPERMINT OIL, EUCALYPTUS OIL, WILLOW BARK EXTRACT, HEMP EXTRACT (AERIAL PARTS, 222MG)
| HOLUS RELIEF GEL, 1.7 OZ
menthol, camphor gel |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Nemadji Management, LLC (100279564) |
| Registrant - Nemadji Management, LLC (100279564) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nemadji Management, LLC | 100279564 | manufacture(73278-202) | |