BSS PLUS- balanced salt solution enriched with bicarbonate, dextrose, and glutathione kit
BSS PLUS by
Drug Labeling and Warnings
BSS PLUS by is a Prescription medication manufactured, distributed, or labeled by Alcon Laboratories, Inc., Alcon Research LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
DESCRIPTION: BSS PLUS® is a sterile intraocular irrigating solution for use during all intraocular surgical procedures, including those requiring a relatively long intraocular perfusion time (e.g., pars plana vitrectomy, phacoemulsification, extracapsular cataract extraction/lens aspiration, anterior segment reconstruction, etc.). The solution does not contain a preservative and should be prepared just prior to use in surgery.
Part I: Part I is a sterile 480 mL solution in a 500 mL single-dose bottle to which the Part II concentrate is added. Each mL of Part I contains: sodium chloride 7.44 mg, potassium chloride 0.395 mg, dibasic sodium phosphate 0.433 mg, sodium bicarbonate 2.19 mg, hydrochloric acid and/or sodium hydroxide (to adjust pH), in water for injection.
Part II: Part II is a sterile concentrate in a 20 mL single-dose vial for addition to Part I. Each mL of Part II contains: calcium chloride dihydrate 3.85 mg, magnesium chloride hexahydrate 5 mg, dextrose 23 mg, glutathione disulfide (oxidized glutathione) 4.6 mg, in water for injection.
After addition of BSS PLUS Part II to the Part I bottle, each mL of the reconstituted product contains: sodium chloride 7.14 mg, potassium chloride 0.38 mg, calcium chloride dihydrate 0.154 mg, magnesium chloride hexahydrate 0.2 mg, dibasic sodium phosphate 0.42 mg, sodium bicarbonate 2.1 mg, dextrose 0.92 mg, glutathione disulfide (oxidized glutathione) 0.184 mg, hydrochloric acid and/or sodium hydroxide (to adjust pH), in water for injection.
The reconstituted product has a pH of approximately 7.4. Osmolality is approximately 305 mOsm.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: None of the components of BSS PLUS are foreign to the eye, and BSS PLUS has no pharmacological action. Human perfused cornea studies have shown BSS PLUS to be an effective irrigation solution for providing corneal detumescence and maintaining corneal endothelial integrity during intraocular perfusion. An in vivo study in rabbits has shown that BSS PLUS is more suitable than normal saline or Balanced Salt Solution for intravitreal irrigation because BSS PLUS contains the appropriate bicarbonate, pH, and ionic composition necessary for the maintenance of normal retinal electrical activity. Human in vivo studies have demonstrated BSS PLUS to be safe and effective when used during surgical procedures such as pars plana vitrectomy, phacoemulsification, cataract extraction/lens aspiration, anterior segment reconstruction. No differences have been observed between adults and pediatric patients following use of this drug product.
- INDICATIONS & USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
PRECAUTIONS: DO NOT USE BSS PLUS UNTIL PART I IS FULLY RECONSTITUTED WITH PART II. Discard unused contents. BSS PLUS does not contain a preservative; therefore, do not use this container for more than one patient. DISCARD ANY UNUSED PORTION SIX HOURS AFTER PREPARATION. Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed.
There have been reports of corneal clouding or edema following ocular surgery in which BSS PLUS was used as an irrigating solution. As in all surgical procedures appropriate measures should be taken to minimize trauma to the cornea and other ocular tissues.
-
SPL UNCLASSIFIED SECTION
Preparation: Reconstitute BSS PLUS® Intraocular Irrigating Solution just prior to use in surgery. Follow the same strict aseptic procedures in the reconstitution of BSS PLUS as is used for intravenous additives. Remove the blue flip-off seal from the BSS PLUS Part I (480 mL) bottle. Remove the blue flip-off seal from the BSS PLUS Part II (20 mL) vial. Clean and disinfect the rubber stoppers on both containers by using sterile alcohol wipes. Transfer the contents of the Part II vial to the Part I bottle using a BSS PLUS Vacuum Transfer Device (provided). An alternative method of solution transfer may be accomplished by using a 20 mL syringe to remove the Part II solution from the vial and transferring exactly 20 mL to the Part I container through the outer target area of the rubber stopper. An excess volume of Part II is provided in each vial. Gently agitate the contents to mix the solution. Place a sterile cap on the bottle. Remove the tear-off portion of the label. Record the time and date of reconstitution and the patient's name on the bottle label.
- GERIATRIC USE
- ADVERSE REACTIONS
- OVERDOSAGE
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: The solution should be used according to the standard technique employed by the operating surgeon. Use an administration set with an air-inlet in the plastic spike since the bottle does not contain a separate airway tube. Follow the directions for the particular administration set to be used. Insert the spike aseptically into the bottle through the center target area of the rubber stopper. Allow the fluid to flow to remove air from the tubing before intraocular irrigation begins. If a second bottle is necessary to complete the surgical procedure, ensure that the vacuum is vented from the second bottle BEFORE attachment to the administration set.
-
HOW SUPPLIED
HOW SUPPLIED: BSS PLUS is supplied in two packages for reconstitution prior to use: a 500 mL glass bottle containing 480 mL (Part I) and a 20 mL glass vial (Part II); both using grey butyl stoppers and aluminum seals with polypropylene flip-off caps. See the PRECAUTIONS section regarding reconstitution of the solution.
NDC: 0065-0800-50.- Remove the blue flip-off seal from the BSS PLUS® Part I (480 mL) bottle. Remove the blue flip-off seal from the BSS PLUS Part II (20 mL) vial. Prepare the stoppers on both parts by using sterile alcohol wipes.
-
Peel open a BSS PLUS Vacuum Transfer Device package (supplied) and remove the sterile transfer spike.
NOTE: This device is vented permitting air to enter vial during solution transfer, thereby preventing the creation of a vacuum inside the vial. An air-inlet filter is provided to protect the system. Do not remove the air-inlet filter. - Remove protector from the white plastic piercing pin.
- Firmly grasp device from behind the flange and insert the white plastic piercing pin into the upright rubber stopper of the BSS PLUS Part II (20 mL) vial.
- Remove guard from filter needle. Firmly grasp vial in the palm of one hand and with thumb and index finger, hold plastic flange against top of vial.
-
Invert vial and immediately insert filter needle into the outer target of the rubber stopper of the BSS PLUS Part I (480 mL) bottle.
(See illustration.) - Fluid will automatically transfer from the vial into the large vacuum bottle unless filter becomes occluded or loss of vacuum occurs. NOTE: An excess amount of BSS PLUS Part II is provided in each vial. A non-transferred solution residual of approximately 0.3 mL can be expected to remain in the vial.
- Immediately remove needle from the BSS PLUS Part I container and discard it after solution transfer has been completed.
- Place a sterile safety cap over the rubber stopper of Part I if the solution is not going to be used immediately. Mix the solution gently until uniform. Peel off the right-hand side of Part I bottle label (fully reconstituted BSS PLUS Solution). Record the patient’s name and the date and time of reconstitution. BSS PLUS Solution is now ready for use.
CAUTION: Reconstituted BSS PLUS Solution must be used within six hours of mixing. Discard any solution which has aged beyond that time. Never use the same bottle of BSS PLUS Solution on more than one patient.
Alternative Transfer Method
If preferred, the contents of the BSS PLUS Part II component may be aspirated with an 18-gauge cannula attached to a 20 mL syringe and then transferred into the Part I bottle.
Alcon®
a Novartis company
Distributed by:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
Revised: November 2015
9012661-1115
- STORAGE AND HANDLING
-
INSTRUCTIONS FOR USE
BSS PLUS®
STERILE INTRAOCULAR IRRIGATING SOLUTION
(balanced salt solution enriched with bicarbonate, dextrose, and glutathione)RECONSTITUTION INSTRUCTIONS
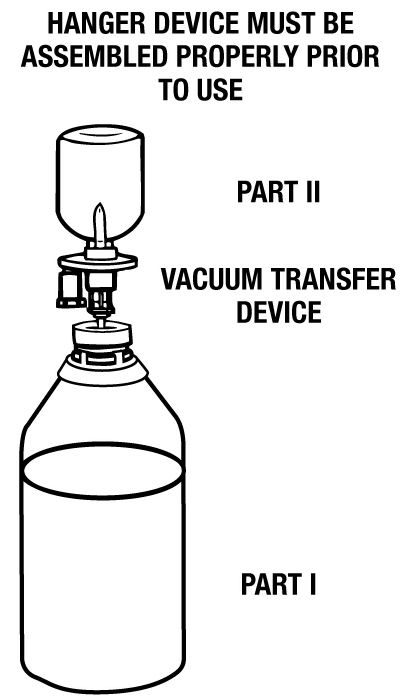
DIRECTIONS: Use Aseptic Technique
- Remove the blue flip-off seal from the BSS PLUS® Part I (480 mL) bottle. Remove the blue flip-off seal from the BSS PLUS Part II (20 mL) vial. Prepare the stoppers on both parts by using sterile alcohol wipes.
- Peel open a BSS PLUS Vacuum Transfer Device package (supplied) and remove the sterile transfer spike.
NOTE: This device is vented permitting air to enter vial during solution transfer, thereby preventing the creation of a vacuum inside the vial. An air-inlet filter is provided to protect the system. Do not remove the air-inlet filter. - Remove protector from the white plastic piercing pin.
- Firmly grasp device from behind the flange and insert the white plastic piercing pin into the upright rubber stopper of the BSS PLUS Part II (20 mL) vial.
- Remove guard from filter needle. Firmly grasp vial in the palm of one hand and with thumb and index finger, hold plastic flange against top of vial.
- Invert vial and immediately insert filter needle into the outer target of the rubber stopper of the BSS PLUS Part I (480 mL) bottle. (See illustration.)
- Fluid will automatically transfer from the vial into the large vacuum bottle unless filter becomes occluded or loss of vacuum occurs. NOTE: An excess amount of BSS PLUS Part II is provided in each vial. A non-transferred solution residual of approximately 0.3 mL can be expected to remain in the vial.
- Immediately remove needle from the BSS PLUS Part I container and discard it after solution transfer has been completed.
- Place a sterile safety cap over the rubber stopper of Part I if the solution is not going to be used immediately. Mix the solution gently until uniform. Peel off the right-hand side of Part I bottle label (fully reconstituted BSS PLUS Solution). Record the patient’s name and the date and time of reconstitution. BSS PLUS Solution is now ready for use.
CAUTION: Reconstituted BSS PLUS Solution must be used within six hours of mixing. Discard any solution which has aged beyond that time. Never use the same bottle of BSS PLUS Solution on more than one patient.
Alternative Transfer Method
If preferred, the contents of the BSS PLUS Part II component may be aspirated with an 18-gauge cannula attached to a 20 mL syringe and then transferred into the Part I bottle.Alcon®
a Novartis company
Distributed by:
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
Revised: November 2015
W9013858-1016
-
PRINCIPAL DISPLAY PANEL
NDC 0065-0800-50
500 mL
Single Patient Use
BSS PLUS®
STERILE INTRAOCULAR IRRIGATING SOLUTION
(balanced salt solution enriched with bicarbonate, dextrose, and glutathione)
After addition of BSS PLUS®– Part II (20 mL), each mL contains: sodium chloride 7.14 mg, potassium chloride 0.38 mg, calcium chloride dihydrate 0.154 mg, magnesium chloride hexahydrate 0.2 mg, dibasic sodium phosphate 0.42 mg, sodium bicarbonate 2.1 mg, dextrose 0.92 mg, glutathione disulfide (oxidized glutathione) 0.184 mg, hydrochloric acid and/or sodium hydroxide (to adjust pH), in water for injection.
pH Approx. 7.4 – Osmolality Approx. 305 mOsm/kg
Rx Only
WARNINGS: FOR IRRIGATION DURING OPHTHALMIC SURGERY ONLY. NOT FOR INJECTION OR INTRAVENOUS INFUSION. DO NOT USE UNLESS PRODUCT IS CLEAR, SEAL IS INTACT, VACUUM IS PRESENT AND CONTAINER IS UNDAMAGED. DO NOT USE IF PRODUCT IS DISCOLORED OR CONTAINS A PRECIPITATE.
PRECAUTIONS: DO NOT USE BSS PLUS UNTIL PART I IS FULLY RECONSTITUTED WITH PART II.
DISCARD UNUSED CONTENTS SIX HOURS AFTER PREPARATION.
DO NOT USE THIS CONTAINER FOR MORE THAN ONE PATIENT.
Reconstitute just prior to use in surgery.
Alcon®
a Novartis company
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
©2002-2003, 2015 Novartis
Patient_____________________
Reconstituted at _________________ AM/PM on_________________
LOT:
EXP.:
SINGLE USE ONLY
NDC 0065-0800-50
480 mL
BSS PLUS® PART I
STERILE SOLUTION
(For Reconstitution by Addition of BSS PLUS® Concentrate Part II, 20 mL)*
Each mL contains: sodium chloride 7.44 mg, potassium chloride 0.395 mg, dibasic sodium phosphate 0.433 mg, sodium bicarbonate 2.19 mg, hydrochloric acid and/or sodium hydroxide (to adjust pH), in water for injection.
Rx Only
Storage: Store at 2° - 25°C (36° - 77°F).
WARNINGS: FOR IRRIGATION DURING OPHTHALMIC SURGERY ONLY AFTER RECONSTITUTION. DO NOT USE UNLESS PRODUCT IS CLEAR, SEAL IS INTACT, VACUUM IS PRESENT AND CONTAINER IS UNDAMAGED.
NOT FOR INJECTION OR INTRAVENOUS INFUSION
*Reconstitute just prior to use in surgery. Remove this part of label after reconstitution. Read the Preparation and Administration sections of the package insert before reconstitution for important directions and precautions.
H14138-1115
NDC 0065-0800-50
20 mL
BSS PLUS®
CONCENTRATE PART II
(Sterile Solution for Reconstitution)
Alcon®
Alcon Laboratories Inc.
Fort Worth, Texas 76134 USA
Each mL contains: calcium chloride dihydrate 3.85 mg, magnesium chloride hexahydrate 5 mg, dextrose 23 mg, glutathione disulfide (oxidized glutathione) 4.6 mg, in water for injection.
For Addition to BSS PLUS® — Part I (480 mL) only.
See Preparation section of package insert.
WARNINGS: Not for injection or intravenous infusion – for reconstitution of irrigation solution only. Do not use unless product is clear, seal is intact and container is undamaged. Do not use if product is discolored or contains a precipitate.
PRECAUTIONS: Discard unused contents. Do not use this container for more than one patient.
Storage: Store at 2°-25°C (36°-77°F).
Rx Only
©2002-2003, 2015 Novartis
H14142-1115
LOT:
EXP.:

BSS Plus®
STERILE IRRIGATING SOLUTION
(balanced salt solution enriched with bicarbonate, dextrose, and glutathione)
Carton Contents:
1 Vial BSS PLUS® Concentrate Part II Sterile Solution for Reconstitution with BSS PLUS Part I Only
1 BSS PLUS Vacuum Transfer Device
1 BSS PLUS Product Package Insert
WARNINGS: For IRRIGATION during ophthalmic surgery only. Not for injection or intravenous infusion. Do not use unless product is clear, seal is intact, vacuum is present and container is undamaged. Do not use if product is discolored or contains a precipitate.
Stare at 2°-35°C (36°-77°F).
Discard prepared solution after six hours.
Alcon®
a Novartis company
Alcon Laboratories Inc. Fort Worth, Texas 76134 USA
© 2002, 2013, 2015 Novartis
PRECAUTIONS: DO NOT USE BSS PLUS® solution UNTIL PART I IS FULLY RECONSTITUTED WITH PART II. Discard unused contents. BSS PLUS does not contain a preservative; therefore, do not use this container for more than one patient. DISCARD ANY UNUSED PORTION SIX HOURS AFTER PREPARATION. Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed. There have been reports of corneal clouding or edema following ocular surgery in which BSS PLUS solution was used as an irrigating solutions. As in all surgical procedures, appropriate measures should be taken to minimize trauma to the cornea and other ocular tissues.
LOT:
EXP:
9012435-0116

-
INGREDIENTS AND APPEARANCE
BSS PLUS
balanced salt solution enriched with bicarbonate, dextrose, and glutathione kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0065-0800 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0065-0800-50 1 in 1 PACKAGE; Type 0: Not a Combination Product 06/28/1982 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, GLASS 480 mL Part 2 1 VIAL, SINGLE-DOSE 20 mL Part 1 of 2 PART 1
balanced salt solution enriched with bicarbonate, dextrose, and glutathione solutionProduct Information Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (Sodium Cation - UNII:LYR4M0NH37) Sodium Chloride 7.44 mg in 1 mL Potassium Chloride (UNII: 660YQ98I10) (Potassium Cation - UNII:295O53K152) Potassium Chloride 0.395 mg in 1 mL Sodium Phosphate, Dibasic (UNII: GR686LBA74) (Sodium Cation - UNII:LYR4M0NH37) Sodium Phosphate, Dibasic 0.433 mg in 1 mL Sodium Bicarbonate (UNII: 8MDF5V39QO) (Sodium Cation - UNII:LYR4M0NH37) Sodium Bicarbonate 2.19 mg in 1 mL Inactive Ingredients Ingredient Name Strength Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 480 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018469 06/28/1982 Part 2 of 2 PART II
calcium chloride, magnesium chloride, dextrose, and glutathione concentrateProduct Information Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Calcium Chloride (UNII: M4I0D6VV5M) (Calcium Cation - UNII:2M83C4R6ZB) Calcium Chloride 3.85 mg in 1 mL Magnesium Chloride (UNII: 02F3473H9O) (Magnesium Cation - UNII:T6V3LHY838) Magnesium Chloride 5 mg in 1 mL Dextrose (UNII: IY9XDZ35W2) (Dextrose - UNII:IY9XDZ35W2) Dextrose 23 mg in 1 mL Oxiglutatione (UNII: ULW86O013H) (Oxiglutatione - UNII:ULW86O013H) Oxiglutatione 4.6 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018469 06/28/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018469 06/28/1982 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0800)
Trademark Results [BSS PLUS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BSS PLUS 73351330 1236020 Live/Registered |
Alcon Laboratories, Inc. 1982-02-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.