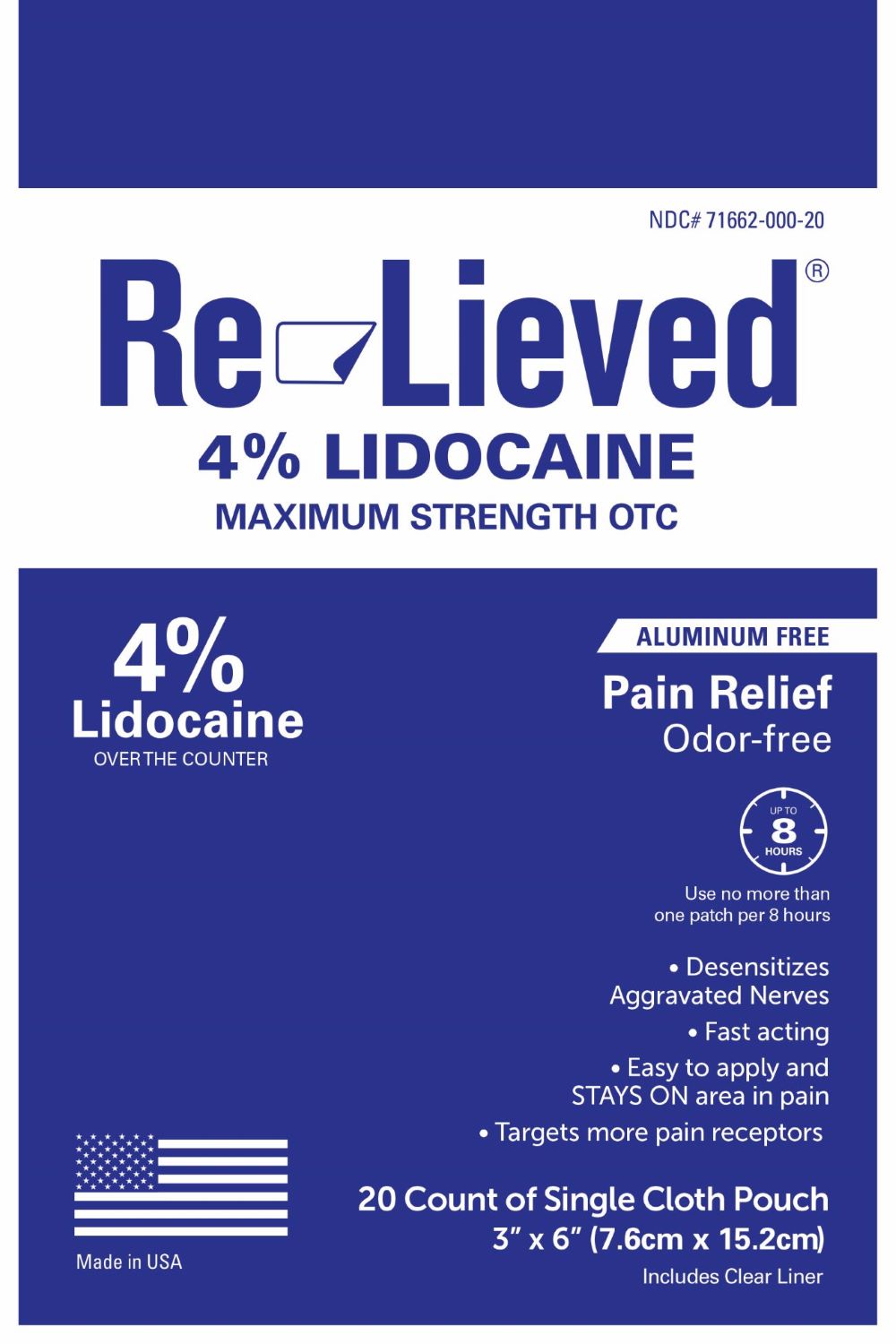
RE-LIEVED LIDOCAINE PATCH- lidocaine patch 4% patch
Re-Lieved Lidocaine Patch by
Drug Labeling and Warnings
Re-Lieved Lidocaine Patch by is a Otc medication manufactured, distributed, or labeled by Transfer Technology, Transfer Technoloy. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Ask your doctor or pharmacist before use if you are
- Stop use or ask a doctor if
- If pregnant or breast feeding
- Keep out of reach of children
- DOSAGE & ADMINISTRATION
- Other information
- Inactive Ingredients
- Re-Lieved Lidocaine 4% 20 Cloth Patches in Indivdual Pouches
-
INGREDIENTS AND APPEARANCE
RE-LIEVED LIDOCAINE PATCH
lidocaine patch 4% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71662-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 mg in 100 mg Inactive Ingredients Ingredient Name Strength ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71662-010-20 4 mg in 1 POUCH; Type 0: Not a Combination Product 03/13/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/13/2023 Labeler - Transfer Technology (037968132) Registrant - Transfer Technology (037968132) Establishment Name Address ID/FEI Business Operations Transfer Technoloy 037968132 manufacture(71662-010)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.