LACTATED RINGERS- sodium chloride, sodium lactate, potassium chloride, and calcium chloride injection, solution
Lactated Ringers by
Drug Labeling and Warnings
Lactated Ringers by is a Prescription medication manufactured, distributed, or labeled by B. Braun Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LACTATED RINGER’S INJECTION safely and effectively. See full prescribing information for LACTATED RINGER’S INJECTION.
LACTATED RINGER’S injection, for intravenous use
Initial U.S. Approval: 1971RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Lactated Ringer’s Injection is indicated for use as a source of water and electrolytes or as an alkalinizing agent in adults and pediatric patients. (1)
DOSAGE AND ADMINISTRATION
- The recommended dosage and duration is based on the patient’s age, weight, clinical condition, and concomitant therapy. (2.1)
- To reduce the risk of air embolism, adhere to the preparation instructions. (2.2, 5.2)
- Lactated Ringer’s Injection is for intravenous use. (2.3)
- Instructions for Intravenous Administration of Plastic Container. (2.4)
- Do not administer Lactated Ringer’s Injection simultaneously with ceftriaxone in neonates (28 days of age or younger) due to serious risks. (2.5)
- See full prescribing information for information dosage considerations, preparation, administration, and drug incompatibilities. (2)
DOSAGE FORMS AND STRENGTHS
- Lactated Ringer’s Injection, USP 1000 mL for intravenous infusion (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Serious Risks with Inappropriate Use with Ceftriaxone: Deaths have occurred in neonates (28 days of age or younger) who received concomitant intravenous calcium-containing solutions with ceftriaxone. In patients older than 28 days, ceftriaxone and Lactated Ringer’s Injection may be administered sequentially if the infusion lines are thoroughly flushed between infusions. (4, 5.1, 8.4)
- Air Embolism: Use a non-vented infusion set or close the vent on a vented set and use a dedicated line without any connections. Pressure infusion is not recommended to increase flow rates, but if necessary, remove all air from the bag prior to initiating infusion. (5.2)
- Hypersensitivity Reactions: Stop the Lactated Ringer’s Injection infusion immediately if signs or symptoms of a hypersensitivity reaction develop. (5.3)
- Potassium Imbalances, Hyponatremia, Hypercalcemia, Fluid Overload, Acid-Base Imbalances, Interference with Interpretation of Serum Lactate Levels in Patients with Severe Metabolic Acidosis: See Full Prescribing Information for risk management recommendations. (5.4, 5.5, 5.6, 5.7, 5.8, 5.9)
ADVERSE REACTIONS
Common adverse reactions include infusion site reactions and symptoms of hypersensitivity reactions (e.g., pruritus, dyspnea, urticaria, rash, cough). (6)
To report SUSPECTED ADVERSE REACTIONS, contact B. Braun Medical Inc. at 1-833-425-1464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Drugs that Affect Electrolyte and/or Fluid Balance: Avoid concomitant use. If concomitant use cannot be avoided, closely monitor electrolyte concentrations and fluid balance. (7.1)
- Lithium: Avoid concomitant use. If concomitant use is unavoidable monitor serum lithium concentrations more frequently. (7.2)
- Digoxin: Consider reducing the volume or rate of Lactated Ringer’s Injection due to the increased risk of digoxin toxicity with calcium-containing solutions. (7.3)
- Drugs with pH-Dependent Renal Elimination: Renal clearance of acidic drugs may be increased. In contrast, renal clearance of alkaline drugs may be decreased. (7.4)
USE IN SPECIFIC POPULATIONS
- Closely monitor plasma electrolyte concentrations in young pediatric patients with immature kidney function. (8.4)
- Geriatric patients are more likely to have decreased renal function. Consider monitoring renal function and starting the infusion at the low end of the dosing range. (8.5)
- Avoid in patients with severe renal impairment. (8.6)
- Patients with severe hepatic impairment may have impaired lactate metabolism. Closely monitor serum lactate levels and acid-base status. (8.7)
Revised: 10/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Considerations
2.2 Important Preparation Instructions
2.3 Important Administration Instructions
2.4 Drug Incompatibilities
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Risk with Concomitant Use with Ceftriaxone
5.2 Air Embolism
5.3 Hypersensitivity Reactions
5.4 Potassium Imbalances
5.5 Hyponatremia
5.6 Hypercalcemia
5.7 Fluid Overload
5.8 Acid/Base Imbalances
5.9 Interference of Lactated Ringer’s Injection with Interpretation of Serum Lactate Levels in Patients with Severe Metabolic Acidosis
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Drugs that Affect Electrolyte and/or Fluid Balance
7.2 Lithium
7.3 Digoxin
7.4 Drugs with pH-Dependent Renal Elimination
7.5 Interference of Lactated Ringer’s Injection with Interpretation of Serum Lactate Levels in Patients with Severe Metabolic Acidosis
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Considerations
The recommended dosage and duration of Lactated Ringer’s Injection is directed by a physician based on the patient’s age, weight, clinical condition, and concomitant therapy. Evaluate the patient’s clinical status and monitor changes in electrolyte concentrations especially during prolonged use of Lactated Ringer’s Injection to optimize clinical status.
2.2 Important Preparation Instructions
Visually inspect the Lactated Ringer’s Injection solution for particulate matter and discoloration. Do not administer Lactated Ringer’s Injection unless the solution is clear and the container and seals are intact.
If additives are determined to be compatible with Lactated Ringer’s Injection then using aseptic technique, mix thoroughly; do not store solutions containing additives. After mixing, do not use if there is discoloration or formation of precipitates.
To reduce the risk of air embolism, adhere to the following Lactated Ringer’s Injection preparation instructions [see Warnings and Precautions (5.2)]:
- Use a non-vented infusion set or close the vent on a vented set.
- Use a dedicated line without any connections (do not connect flexible containers in series).
- The use of pressure infusion is not recommended as a method to increase flow rates. However, if pressure infusion is required, ensure that any air within the bag is fully evacuated prior to initiation of infusion. If using a pumping device to administer Lactated Ringer’s Injection, turn off the pump before the container is empty.
Preparation Instructions
1. Check for minute leaks by squeezing solution container firmly. If leaks are found, discard solution as sterility may be impaired. If supplemental medication is desired, follow directions below before preparing for administration [see Dosage and Administration (2.3)].
2. Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
3. Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter. Any container which is suspect should not be used.
4. If supplemental medication is desired, follow directions below [see Dosage and Administration (2.3)].Preparation for Administration
1. Remove plastic protector from sterile set port at bottom of container.
2. Attach administration set according to its accompanying directions.2.3 Important Administration Instructions
Lactated Ringer’s Injection is for intravenous use.
Use immediately after opening the container. Discard the unused portion. Some additives may be incompatible [see Dosage and Administration (2.4)].
To Add Medication Before Solution Administration
1. Prepare medication site by removing the additive port closure. Swab the exposed medication site before puncturing.
2. Using syringe with 18-22 gauge needle, puncture medication port and inner diaphragm and inject.
3. Squeeze and tap ports while ports are upright and mix solution and medication thoroughly.To Add Medication During Solution Administration
1. Close clamp on the set.
2. Prepare medication site by removing the additive port closure. Swab the exposed medication site before puncturing.
3. Using syringe with 18-22 gauge needle of appropriate length (at least 5/8 inch), puncture resealable medication port and inner diaphragm and inject.
4. Remove container from IV pole and/or turn to an upright position.
5. Evacuate both ports by tapping and squeezing them while container is in the upright position.
6. Mix solution and medication thoroughly.
7. Return container to in use position and continue administration.2.4 Drug Incompatibilities
Do not administer Lactated Ringer’s Injection simultaneously with ceftriaxone in neonates (28 days of age or younger) due to serious risks [see Contraindications (4) and Warnings and Precautions (5.1)]. However, in patients older than 28 days, ceftriaxone and Lactated Ringer’s Injection may be administered sequentially if the infusion lines are thoroughly flushed between infusions with a compatible fluid [see Warnings and Precautions (5.1)].
Do not administer Lactated Ringer’s Injection simultaneously with citrate anticoagulated/preserved blood through the same administration set because of the likelihood of coagulation precipitated by the calcium content of Lactated Ringer’s Injection.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Lactated Ringer’s Injection, USP is contraindicated in:
- Neonates (28 days of age or younger) who are receiving concomitant treatment with ceftriaxone, even if separate infusion lines are used, due to the risk of fatal ceftriaxone-calcium salt precipitation in the neonate’s bloodstream [see Warnings and Precautions (5.1) and Specific Populations (8.4)].
- Patients with known hypersensitivity to any of the components of Lactated Ringer’s Injection [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Risk with Concomitant Use with Ceftriaxone
Precipitation of ceftriaxone-calcium can occur when ceftriaxone is mixed with calcium-containing solutions, such as Lactated Ringer’s Injection, in the same intravenous administration line. Deaths have occurred in neonates (28 days of age or younger) who received concomitant intravenous calcium-containing solutions with ceftriaxone resulting from calcium-ceftriaxone precipitates in the lungs and kidneys, even when separate infusion lines were used:
Lactated Ringer’s Injection is contraindicated in neonates who receive ceftriaxone [see Contraindications (4), Use in Specific Populations (8.4)]. However, in patients older than 28 days, ceftriaxone and Lactated Ringer’s Injection may be administered sequentially if the infusion lines are thoroughly flushed between infusions with a compatible fluid.
5.2 Air Embolism
Cases of air embolism have been reported with pressurized administration of intravenous fluids. Air embolism may result in stroke, organ ischemia and/or infarction, and death.
Use a non-vented infusion set or close the vent on a vented set and use a dedicated line without any connections.
If administration is controlled by a pumping device, care must be taken to discontinue the pumping action before the container is empty.
Pressure infusion is not recommended to increase flow rates, but if necessary, ensure all air is removed from the bag before infusion.
Refrain from applying excessive pressure (>300mmHg) causing distortion to the container such as wringing or twisting. Such handling could result in breakage of the container [see Dosage and Administration (2.2)].
5.3 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with Lactated Ringer’s Injection. Stop the Lactated Ringer’s Injection infusion immediately and treat patient accordingly if signs or symptoms of a hypersensitivity reaction develop. Initiate appropriate treatment as clinically indicated.
5.4 Potassium Imbalances
Hyperkalemia
Potassium-containing solutions, including Lactated Ringer’s Injection, may increase the risk of hyperkalemia. This risk is increased in patients predisposed to hyperkalemia including those with severe renal impairment, acute dehydration, extensive tissue injury or burns, heart failure, or in those using concomitant drugs that are associated with hyperkalemia.
Avoid use of Lactated Ringer’s Injection in patients with, or at increased risk for, hyperkalemia. If use cannot be avoided in these patients, closely monitor serum potassium concentrations.
5.5 Hyponatremia
Lactated Ringer’s Injection may cause hyponatremia. Hyponatremia can lead to acute hyponatremic encephalopathy characterized by headache, nausea, seizures, lethargy and vomiting. The risk of hospital-acquired hyponatremia is increased in younger pediatric patients, geriatric patients, patients treated with diuretics, and patients with cardiac or pulmonary failure or with the syndrome of inappropriate antidiuretic hormone (SIADH) (e.g., postoperative patients, patients concomitantly treated with arginine vasopressin analogs, or certain antiepileptic, psychotropic, or cytotoxic drugs) [see Drug Interactions (7.1), Use in Specific Populations (8.4)].
Avoid Lactated Ringer’s Injection in patients with or at risk for hyponatremia. If use cannot be avoided in these patients, closely monitor serum sodium concentrations.
Rapid correction of hyponatremia may result in serious neurologic complications such as osmotic demyelination syndrome (ODS). To avoid complications, monitor serum sodium and chloride concentrations, fluid status, acid-base balance, and neurologic status.
5.6 Hypercalcemia
Lactated Ringer’s Injection contains calcium salts and may cause hypercalcemia. Avoid administration of Lactated Ringer’s Injection in patients with hypercalcemia, those with calcium-containing renal calculi or history of such calculi, those with conditions predisposing to hypercalcemia, or treated with concomitant thiazide diuretics or vitamin D.
5.7 Fluid Overload
Depending on the administered volume and the infusion rate, administration of Lactated Ringer’s Injection can cause fluid overload, including pulmonary edema.
Avoid Lactated Ringer’s Injection in patients at risk for fluid and/or solute overload including patients with severe renal impairment. If use cannot be avoided in these patients, monitor fluid balance, electrolyte concentrations and acid base balance, especially during prolonged use.
5.8 Acid/Base Imbalances
Because lactate is metabolized to bicarbonate, administration of Lactated Ringer’s Injection may result in, or worsen, metabolic alkalosis. Closely monitor the acid-base balance in patients with, or at risk of, alkalosis.
In patients with severe hepatic impairment, decreased lactate metabolism may result in worsening anion gap metabolic acidosis. Avoid Lactated Ringer’s Injection in patients with severe hepatic impairment. If use cannot be avoided in these patients, closely monitor serum bicarbonate levels.
5.9 Interference of Lactated Ringer’s Injection with Interpretation of Serum Lactate Levels in Patients with Severe Metabolic Acidosis
Administration of Lactated Ringer’s Injection may result in interference with the interpretation of serum lactate levels in patients with severe metabolic acidosis [see Drug Interactions (7.5)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
Serious Risk with Concomitant Use with Ceftriaxone [see Warnings and Precautions (5.1)]
Air Embolism [see Warnings and Precautions (5.2)]
Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
Potassium Imbalances [see Warnings and Precautions (5.4)]
Hyponatremia [see Warnings and Precautions (5.5)]
Hypercalcemia [see Warnings and Precautions (5.6)]
Fluid Overload [see Warnings and Precautions (5.7)]
Acid/Base Imbalances [see Warnings and Precautions (5.8)]
The following adverse reactions have been identified during postapproval use of Lactated Ringer’s Products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
General Disorders and Administration Site Conditions:
Phlebitis, extravasation, infusion site inflammation, infusion site swelling, infusion site rash, infusion site pruritus, infusion site erythema, infusion site pain, infusion site burning, and infusion site hypoaesthesia.
Hypersensitivity Reactions and Infusion Reactions:
Angioedema, chest pain/discomfort, bradycardia or tachycardia, hypotension, respiratory distress, bronchospasm, dyspnea, cough, urticaria, rash, pruritus, erythema, flushing, throat irritation, paresthesia, oral hypoesthesia, dysgeusia, nausea, anxiety, pyrexia, headache, laryngeal edema, sneezing, and injection site infection.
Metabolism and Nutrition Disorders:
Hyperkalemia, hyponatremia, and hypervolemia.
Nervous System Disorders:
Hyponatremic encephalopathy.
-
7 DRUG INTERACTIONS
7.1 Drugs that Affect Electrolyte and/or Fluid Balance
Hyperkalemia
Administration of Lactated Ringer’s Injection to patients concomitantly treated or recently treated with drugs that are associated with hyperkalemia increases the risk of severe and potentially fatal hyperkalemia, especially in the presence of other hyperkalemia risk factors. Avoid use of Lactated Ringer’s Injection in patients receiving drugs that are associated with hyperkalemia (e.g., potassium-sparing diuretics, ACE inhibitors, angiotensin II receptor antagonists, or calcineurin inhibitors). If concomitant use cannot be avoided, closely monitor serum potassium concentrations during concomitant use [see Warnings and Precautions (5.4)].
Hyponatremia
Administration of Lactated Ringer’s Injection to patients treated concomitantly with drugs associated with hyponatremia may increase the risk of developing hyponatremia. These drugs include diuretics and those that cause SIADH (e.g. arginine vasopressin analogs, certain antiepileptic, psychotropic, or cytotoxic drugs). Avoid use of Lactated Ringer’s Injection in patients receiving such drugs. If use cannot be avoided, closely monitor serum sodium concentrations during concomitant use [see Warnings and Precautions (5.5)].
Hypercalcemia
Avoid the use of Lactated Ringer’s Injection in patients treated with thiazide diuretics or vitamin D because these drugs can increase the risk of hypercalcemia. If use cannot be avoided, closely monitor serum calcium concentrations during concomitant use [see Warnings and Precautions (5.6)].
Hypernatremia and Fluid Retention
Administration of Lactated Ringer’s Injection to patients treated concomitantly with drugs associated with sodium and fluid retention (e.g., corticosteroids or corticotropin) may increase the risk of hypernatremia and volume overload. Avoid use of Lactated Ringer’s Injection in patients receiving such drugs. If use cannot be avoided, closely monitor serum electrolytes, fluid balance, and acid-base balance during concomitant use.
7.2 Lithium
Renal sodium and lithium clearance may be increased during concomitant use of Lactated Ringer’s Injection and lithium and may result in decreased lithium concentrations. Avoid use of Lactated Ringer’s Injection in patients receiving lithium. If use cannot be avoided, increase the frequency of monitoring of serum lithium concentrations during concomitant use.
7.3 Digoxin
Administration of calcium via use of Lactated Ringer’s Injection may increase digoxin’s effects and lead to digoxin toxicity including serious or fatal cardiac arrhythmias. In digoxin-treated patients, consider reducing the volume and/or rate of Lactated Ringer’s Injection administration.
7.4 Drugs with pH-Dependent Renal Elimination
Due to the alkalinizing action of lactate (formation of bicarbonate), Lactated Ringer’s Injection may interfere with the elimination of drugs with pH-dependent renal elimination. Renal clearance of alkaline drugs may be decreased. In contrast, renal clearance of acidic drugs may be increased.
7.5 Interference of Lactated Ringer’s Injection with Interpretation of Serum Lactate Levels in Patients with Severe Metabolic Acidosis
Because administration of Lactated Ringer’s Injection may interfere with the interpretation of serum lactate levels in patients with severe metabolic acidosis, assessment of the patient’s clinical status should not solely rely on the measurement of serum lactate.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Lactated Ringer’s as a source of water and electrolytes has been used for decades during labor and delivery. Although there are no reports of use of Lactated Ringer’s in other stages of pregnancy, exposure during pregnancy is not expected to cause major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with this drug.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
Lactated Ringer’s as a source of water and electrolytes has been used for decades and is not expected to cause harm to a breastfed infant. There are no data on the presence of Lactated Ringer’s Injection in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Lactated Ringer’s Injection and any potential adverse effects on the breastfed infant from Lactated Ringer’s Injection or from the underlying maternal condition.
8.4 Pediatric Use
Lactated Ringer’s Injection is contraindicated in neonates (28 days of age or younger) who are receiving ceftriaxone due to reported deaths that occurred when neonates received ceftriaxone and intravenous calcium-containing solutions concomitantly [see Warnings and Precautions (5.1)].
The safety and effectiveness of Lactated Ringer’s Injection for use as a source of water and electrolytes or as an alkalinizing agent have been established in pediatric patients of all ages, including neonates.
Closely monitor plasma electrolyte concentrations in young pediatric patients with immature kidney function who may have decreased ability to maintain fluid and electrolyte balance [see Warnings and Precautions (5.4, 5.5, 5.8, 5.9)]. Administration of a lactate-containing intravenous solution, including Lactated Ringer’s Injection to pediatric patients should account for liver and kidney maturation (the kidney function affects the biotransformation and renal excretion of lactate) [see Warnings and Precautions (5.9)].
8.5 Geriatric Use
Geriatric patients treated with Lactated Ringer’s Injection are at increased risk of developing electrolyte imbalances. Lactated Ringer’s Injection is substantially excreted by the kidney, and the risk of adverse reactions to Lactated Ringer’s Injection may be greater in patients with renal impairment than in patients with normal renal function. Because geriatric patients are more likely to have decreased renal function, consider monitoring renal function in geriatric patients and consider starting the infusion at the low end of the dosing range.
8.6 Renal Impairment
Administration of Lactated Ringer’s Injection to patients with or at risk of severe renal impairment, may result in hyperkalemia and/or fluid overload [see Warnings and Precautions (5.4, 5.7, 5.9)]. Avoid Lactated Ringer’s Injection in patients with severe renal impairment. If use cannot be avoided in such patients, monitor for development of these adverse reactions.
-
10 OVERDOSAGE
Excessive administration of Lactated Ringer’s Injection can cause:
- Hyperkalemia and hypernatremia, especially in patients with severe renal impairment.
- Fluid overload (which can lead to pulmonary and/or peripheral edema).
- Metabolic alkalosis with or without hypokalemia.
- Loss of bicarbonate with an acidifying effect
- Hypercalcemia
Overdose interventions include Lactated Ringer’s Injection discontinuation, treatment of hyperkalemia, and close monitoring of fluid balance, electrolyte concentrations, and acid-base balance [see Warnings and Precautions (5.4, 5.5, 5.6, 5.7, 5.8)].
-
11 DESCRIPTION
Lactated Ringer’s Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in a single-dose container intended for intravenous administration.
Composition, osmolarity, pH, ionic concentration and caloric content are shown in Table 1.
- * Normal physiologic osmolarity range is approximately 280 to 310 mOsmol/L.
- † pH may be adjusted with Hydrochloric Acid NF or Sodium Hydroxide NF.
Table 1
Size (mL)Composition (g/L)
Osmolarity*
(mOsmol/L)
(calc)
pH†Ionic Concentration
(mEq/L)
Caloric Content (kcal/L)Sodium
Chloride,
USPSodium
LactatePotassium
Chloride,
USPCalcium
Chloride,
USPSodium Potassium Calcium Chloride Lactate Lactated
Ringer’s
Injection, USP1000 6 3.1 0.3 0.2 274 6.2
(6.0 to
7.5)130 4 3 109 28 9
The chemical name, structural formula, and molecular weight of the active ingredients are shown in Table 2.
Table 2 The formulas of the active ingredients are:
Ingredients Molecular Formula Molecular Weight Sodium Chloride USP Na+CI− 58.44 Sodium Lactate 
112.06 Potassium Chloride USP K+CI− 74.55 Calcium Chloride Dihydrate USP 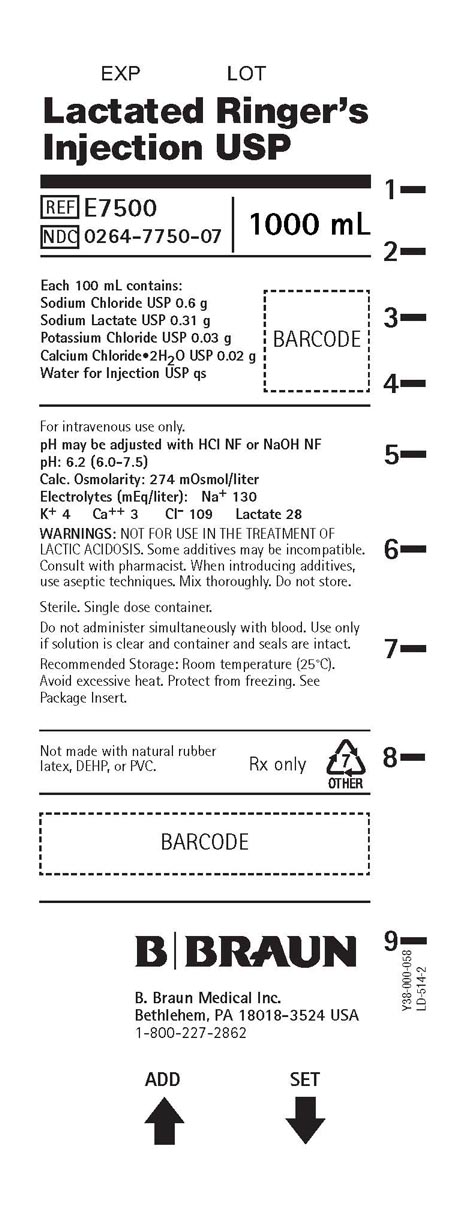
147.02 Not made with natural rubber latex, PVC or DEHP.
The plastic container is made from a homogenous blend of polypropylene and thermoplastic modifier developed for parenteral drugs. The container is a closed system and is not dependent upon entry of external air during administration.
The closure system has two ports; one for the administration set and the other is a medication addition site. Each port has a tamper evident cover [see Dosage and Administration (2.3)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lactated Ringer’s Injection is a source of water, electrolytes, and produces an alkalinizing effect.
- Sodium, the major cation of the extracellular fluid, functions primarily in the control of water distribution, fluid balance, and osmotic pressure of body fluids. Sodium is also associated with chloride and bicarbonate in the regulation of the acid-base equilibrium of body fluid.
- Potassium, the principal cation of intracellular fluid, participates in carbohydrate utilization and protein synthesis and is critical in the regulation of nerve conduction and muscle contraction, particularly in the heart.
- Chloride, the major extracellular anion, closely follows the metabolism of sodium, and changes in the acid-base balance of the body are reflected by changes in the chloride concentration.
- Calcium, an important cation, provides the framework of bones and teeth in the form of calcium phosphate and calcium carbonate. In the ionized form, calcium is essential for the functional mechanism of the clotting of blood, normal cardiac function, and regulation of neuromuscular irritability.
- Sodium lactate provides sodium and lactate ions. The lactate anion is in equilibrium with pyruvate and has an alkalinizing effect resulting from simultaneous removal by the liver of lactate and hydrogen ions. The sodium ion combines with bicarbonate ion produced from carbon dioxide of the body and thus retains bicarbonate to combat metabolic acidosis (bicarbonate deficiency).
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of Lactated Ringer’s Injection have not been fully characterized.
12.3 Pharmacokinetics
Elimination
Metabolism/Excretion
Potassium: Normally about 80 to 90% of the potassium intake is excreted in the urine; the remainder is excreted in feces and to a smaller extent, in perspiration.
Sodium and Chloride: The distribution and excretion of sodium (Na+) and chloride (Cl−) are largely under the control of the kidney which maintains a balance between intake and output.
Lactate: In the liver, lactate is metabolized to carbon dioxide and water by oxidative metabolism and consumption of hydrogen cations.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Lactated Ringer’s Injection, USP is a clear, colorless solution packaged in a single-dose container.
Lactated Ringer’s Injection, USP is supplied sterile and nonpyrogenic in plastic containers packaged 12 per case.
It is available in the following presentation:
NDC REF Size 0264-7750-07 E7500 1000 mL Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature.] Minimize exposure of Lactated Ringer’s Injection, USP to heat. Avoid excessive heat. Protect from freezing.
- SPL UNCLASSIFIED SECTION
-
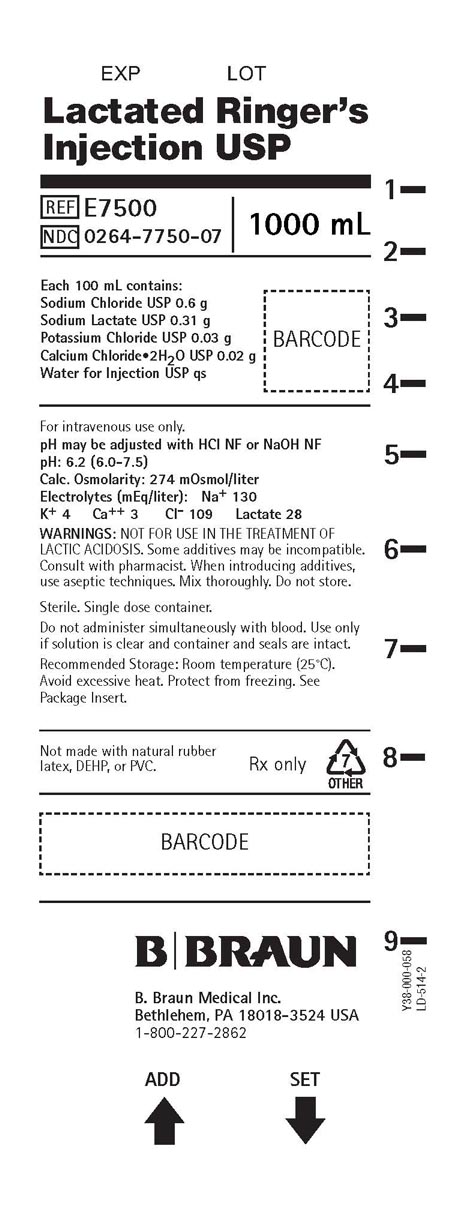
PRINCIPAL DISPLAY PANEL - 1000 ML CONTAINER LABEL
Lactated Ringer’s
Injection USPREF E7500
NDC 0264-7750-07
1000 mL
Each 100 mL contains:
Sodium Chloride USP 0.6 g
Sodium Lactate USP 0.31 g
Potassium Chloride USP 0.03 g
Calcium Chloride2H2O USP 0.02 g
Water for Injection USP qsFor intravenous use only.
pH may be adjusted with HCl NF or NaOH NF
pH: 6.2 (6.0-7.5)
Calc. Osmolarity: 274 mOsmol/liter
Electrolytes (mEq/liter): Na+ 130
K+ 4 Ca++ 3 Cl- 109 Lactate 28WARNINGS: NOT FOR USE IN THE TREATMENT OF
LACTIC ACIDOSIS. Some additives may be incompatible.
Consult with pharmacist. When introducing additives,
use aseptic techniques. Mix thoroughly. Do not store.Sterile. Single dose container.
Do not administer simultaneously with blood. Use only
if solution is clear and container and seals are intact.Recommended Storage: Room temperature (25°C).
Avoid excessive heat. Protect from freezing. See
Package Insert.Not made with natural rubber
latex, DEHP, or PVC.Rx only

B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Y38-000-058
LD-514-2ADD SET
-
INGREDIENTS AND APPEARANCE
LACTATED RINGERS
sodium chloride, sodium lactate, potassium chloride, and calcium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7750 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.6 g in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (LACTIC ACID - UNII:33X04XA5AT, SODIUM CATION - UNII:LYR4M0NH37) SODIUM LACTATE 0.31 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.03 g in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 0.02 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7750-07 12 in 1 CASE 04/26/2024 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019632 04/26/2024 Labeler - B. Braun Medical Inc. (002397347)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.