BIOGLO- fluorescein sodium strip
BioGlo by
Drug Labeling and Warnings
BioGlo by is a Prescription medication manufactured, distributed, or labeled by HUB Pharmaceuticals, Inc., OMNI LENS PRIVATE LIMITED, Madhu Instruments Pvt.Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Product Facts
- INDICATIONS:
-
DIRECTIONS FOR USE:
To insure full fluorescence and patient comfort, the BioGlo impregnated tip should be moistened before application. One or two drops of sterile irrigating or saline solution should be used for this purpose. Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
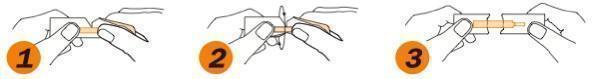
- Grasp tabs between thumbs & index fingers
- Gently pull tabs apart
- Remove strip taking care to touch only the white portion of the strip
- STORAGE:
- NOTE:
- KEEP OUT OF REACH OF CHILDREN
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
- Representative Packaging:
-
INGREDIENTS AND APPEARANCE
BIOGLO
fluorescein sodium stripProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17238-900 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUORESCEIN SODIUM (UNII: 93X55PE38X) (FLUORESCEIN - UNII:TPY09G7XIR) FLUORESCEIN SODIUM 1 mg in 1 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17238-900-11 100 in 1 BOX 04/01/2018 1 NDC: 17238-900-99 1 mg in 1 PACKET; Type 0: Not a Combination Product 2 NDC: 17238-900-30 300 in 1 BOX 04/01/2018 2 NDC: 17238-900-99 1 mg in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/01/2012 Labeler - HUB Pharmaceuticals, Inc. (611747945) Establishment Name Address ID/FEI Business Operations OMNI LENS PRIVATE LIMITED 862170057 manufacture(17238-900) Establishment Name Address ID/FEI Business Operations Madhu Instruments Pvt.Ltd. 915827852 manufacture(17238-900)
Trademark Results [BioGlo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIOGLO 88134998 5739733 Live/Registered |
Trygve Enterprises LLC 2018-09-27 |
 BIOGLO 86394737 4727311 Live/Registered |
Hub Pharmaceuticals, LLC 2014-09-15 |
 BIOGLO 77894877 not registered Dead/Abandoned |
COSWAY (M) SDN. BHD. (Company No. 50118-A) 2009-12-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


