ATROPINE SULFATE solution/ drops
Atropine Sulfate by
Drug Labeling and Warnings
Atropine Sulfate by is a Prescription medication manufactured, distributed, or labeled by NorthStar RxLLC, Mankind Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ATROPINE SULFATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for ATROPINE SULFATE OPHTHALMIC SOLUTION.
ATROPINE SULFATE OPHTHALMIC SOLUTION, 1%,
for topical ophthalmic use
Initial U.S. Approval: 1960INDICATIONS AND USAGE
Atropine is an anticholinergic indicated in adults and pediatric patients aged three (3) months and older for: (1)
- Cycloplegia (1.1)
- Mydriasis (1.2)
- Penalization of the healthy eye in the treatment of amblyopia (1.3)
DOSAGE AND ADMINISTRATION
- Apply 1 drop topically to the cul-de-sac of the conjunctiva, forty minutes prior to the intended maximal dilation time. (2)
- In adults and pediatric patients aged 3 years and older, doses may be repeated up to twice daily as needed. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution: 1% atropine sulfate (3)
CONTRAINDICATIONS
Hypersensitivity or allergic reaction to any ingredient in formulation. (4)
WARNINGS AND PRECAUTIONS
- Photophobia and blurred vision due to pupil unresponsiveness and cycloplegia may last up to 2 weeks. (5.1)
- Risk of blood pressure increase from systemic absorption (5.2)
ADVERSE REACTIONS
Most common adverse reactions that have been reported are eye pain and stinging on administration, blurred vision, photophobia, decreased lacrimation, increased heart rate and blood pressure. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Northstar Rx LLC at 1-800-206-7821 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
The use of atropine and monoamine oxidase inhibitors (MAOI) is generally not recommended because of the potential to precipitate hypertensive crisis. (7)
USE IN SPECIFIC POPULATIONS
Should only be used in pregnant women if clearly needed. (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Cycloplegia
1.2 Mydriasis
1.3 Penalization of the Healthy Eye in the Treatment of Amblyopia
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Photophobia and Blurred Vision
5.2 Elevation of Blood Pressure
5.3 Risk of Contamination
6 ADVERSE REACTIONS
6.1 Ocular Adverse Reactions
6.2 Systemic Adverse Reactions
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase Inhibitors (MAOI)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Photophobia and Blurred Vision
Photophobia and blurred vision due to pupil unresponsiveness and cycloplegia may last up to 2 weeks.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Photophobia and Blurred Vision [See Warnings and Precautions (5.1)]
- Elevation in Blood Pressure [See Warnings and Precautions (5.2)]
The following adverse reactions were identified in clinical studies or postmarketing reports following use of atropine sulfate ophthalmic solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
6.1 Ocular Adverse Reactions
Eye pain and stinging occurs upon instillation. Other commonly occurring adverse reactions include, blurred vision, photophobia, superficial keratitis and decreased lacrimation. Allergic reactions such as papillary conjunctivitis, contact dermatitis, and lid edema may also occur less commonly.
6.2 Systemic Adverse Reactions
Systemic effects of atropine are related to its anti-muscarinic activity. Systemic adverse events reported include dryness of skin, mouth, and throat from decreased secretions from mucus membranes; restlessness, irritability or delirium from stimulation of the central nervous system; tachycardia; flushed skin of the face and neck.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There are no adequate and well-controlled studies of atropine sulfate administration in pregnant women to inform a drug-associated risk. Adequate animal development and reproduction studies have not been conducted with atropine sulfate. In humans, 1% atropine sulfate is systemically bioavailable following topical ocular administration [see Clinical Pharmacology (12.3)].
Atropine Sulfate Ophthalmic Solution 1% should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus.
8.2 Lactation
There is no information to inform risk regarding the presence of atropine in human milk following ocular administration of atropine sulfate to the mother. The effects on breastfed infants and the effects on milk production are also unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for atropine sulfate and any potential adverse effects on the breastfed child from atropine sulfate.
8.4 Pediatric Use
The safety and effectiveness of Atropine Sulfate Ophthalmic Solution 1% have been established in pediatric patients aged 3 months and older. Use of Atropine Sulfate Ophthalmic Solution 1% is supported by evidence from adequate and well-controlled trials with additional safety data from published literature.
Due to the potential for systemic absorption, the use of Atropine Sulfate Ophthalmic Solution 1% in pediatric patients aged 3 months to 3 years should be limited to no more than one drop per eye per day.
Use in pediatric patients younger than 3 months of age is not recommended.
-
10 OVERDOSAGE
In the event of accidental ingestion or toxic overdosage with atropine sulfate ophthalmic solution, supportive care may include a short acting barbiturate or diazepam as needed to control marked excitement and convulsions. Large doses for sedation should be avoided because central depressant action may coincide with the depression occurring late in atropine poisoning. Central stimulants are not recommended.
Physostigmine, given by slow intravenous injection of 1 to 4 mg (0.5 to 1 mg in pediatric populations), rapidly abolishes delirium and coma caused by large doses of atropine. Since physostigmine is rapidly destroyed, the patient may again lapse into coma after one to two hours, and repeated doses may be required.
Artificial respiration with oxygen may be necessary. Cooling measures may be needed to help to reduce fever, especially in pediatric populations.
The fatal adult dose of atropine is not known. In pediatric patients, 10 mg or less may be fatal.
-
11 DESCRIPTION
Atropine Sulfate Ophthalmic Solution, USP 1% is a sterile topical anticholinergic for ophthalmic use. The active ingredient is represented by the chemical structure:
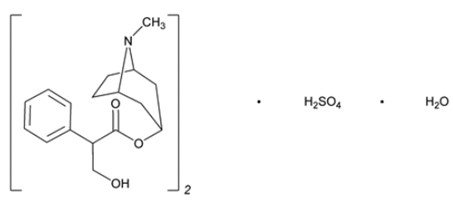
Chemical Name: Benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo[3.2.1.]oct-3-yl ester, endo –(±)-, sulfate (2:1) (salt), monohydrate.
Molecular Formula: (C17H23NO3)2 H2SO4 H2O
Molecular Weight: 694.84 g/mol
Atropine sulfate, USP appears as colorless, almost white to white solid. It is very soluble in water and glacial acetic acid, freely soluble in ethanol (96%) and practically insoluble in diethyl ether.
Each mL of Atropine Sulfate Ophthalmic Solution USP, 1% contains: Active: atropine sulfate, USP 10 mg equivalent to 8.3 mg of atropine. Inactives: benzalkonium chloride 0.1 mg (0.01%), dibasic sodium phosphate anhydrous, edetate disodium dihydrate, hypromellose (2910), monobasic sodium phosphate monohydrate, hydrochloric acid and/or sodium hydroxide may be added to adjust pH (3.5 to 6.0), and water for injection USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Atropine is a reversible antagonist of muscarine-like actions of acetyl-choline and is therefore classified as an antimuscarinic agent. Atropine is relatively selective for muscarinic receptors. Its potency at nicotinic receptors is much lower, and actions at non-muscarinic receptors are generally undetectable clinically. Atropine does not distinguish among the M1, M2, and M3 subgroups of muscarinic receptors.
The pupillary constrictor muscle depends on muscarinic cholinoceptor activation. This activation is blocked by topical atropine resulting in unopposed sympathetic dilator activity and mydriasis. Atropine also weakens the contraction of the ciliary muscle, or cycloplegia. Cycloplegia results in loss of the ability to accommodate such that the eye cannot focus for near vision.
12.2 Pharmacodynamics
The onset of action after administration of atropine sulfate ophthalmic solution 1%, is usually within 40 minutes with maximal effect being reached in about 2 hours. The effect can last for up to 2 weeks in a normal eye.
12.3 Pharmacokinetics
The bioavailability of atropine sulfate ophthalmic solution 1% was assessed in six healthy subjects, 24 to 29 years of age. Subjects received either 0.3 mg atropine sulfate administered as bolus intravenous injection or 0.3 mg administered as 30 μL instilled unilaterally in the cul-de-sac of the eye. Plasma l-hyoscyamine concentrations were determined over selected intervals up to eight hours after dose administration.
The mean bioavailability of topically applied atropine was 63.5 ± 29% (range 19 to 95%) with large inter-individual differences. Mean maximum observed plasma concentration for the ophthalmic solution was 288 ± 73 pg/mL. Maximum concentration was reached in 28 ± 27 min after administration. Terminal half-life of l-hyoscamine was not affected by route of administration and was calculated to be 3 ± 1.2 hours (intravenous) and 2.5 ± 0.8 hours (topical ophthalmic).
In another placebo-controlled study, the systemic exposure to l-hyoscyamine, and the anti-cholinergic effects of atropine were investigated in eight ocular surgery patients 56 to 66 years of age, following single topical ocular 0.4 mg atropine dose (given as 40 microliters of atropine sulfate ophthalmic solution, 1%). The mean (± standard deviation (SD)) Cmax of l-hyoscyamine in these patients was 860 ± 402 pg/mL, achieved within 8 minutes of eyedrop instillation.
Following intravenous administration, the mean (± SD) elimination half-life (t1/2) of atropine was reported to be longer in pediatric subjects under 2 years (6.9 ± 3.3 hours) and in geriatric patients 65 to 75 years (10.0 ± 7.3 hours), compared to in children over 2 years (2.5 ± 1.2 hours) and in adults 16 to 58 years (3.0 ± 0.9 hours). [see Use in Specific Populations (8.4)].
Atropine is destroyed by enzymatic hydrolysis, particularly in the liver; from 13 to 50% is excreted unchanged in the urine. Traces are found in various secretions, including milk. The major metabolites of atropine are noratropine, atropin-n-oxide, tropine, and tropic acid. Atropine readily crosses the placental barrier and enters the fetal circulation, but is not found in amniotic fluid.
Atropine binds poorly (about 44%) to plasma protein, mainly to alpha-1 acid glycoprotein; age has no effect on the serum protein binding of atropine. Atropine binding to α-1 acid glycoprotein was concentration dependent (2 to 20 mcg/mL) and nonlinear in vitro and in vivo. There is no gender effect on the pharmacokinetics of atropine administered by injection.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed to evaluate the carcinogenic potential of atropine sulfate.
Mutagenesis
Atropine sulfate was negative in the salmonella/microsome mutagenicity test.
Impairment of Fertility
Studies to evaluate impairment of fertility have not been conducted.
-
14 CLINICAL STUDIES
Topical administration of Atropine Sulfate Ophthalmic Solution 1% results in cycloplegia and mydriasis which has been demonstrated in several controlled clinical studies in adults and pediatric patients. Maximal mydriasis usually occurs in about 40 minutes and maximal cycloplegia is usually achieved in about 60 to 90 minutes after single administration. Full recovery usually occurs in approximately one week, but may last a couple of weeks.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Atropine Sulfate Ophthalmic Solution, USP 1% is supplied as clear, colorless solution in a three piece white bottle with a natural nozzle and red cap in the following sizes:
NDC: 72603-264-01 2 mL fill in 5 mL bottle
NDC: 72603-264-02 5 mL fill in 5 mL bottle
Storage: Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Keep container tightly closed. After opening, Atropine Sulfate Ophthalmic Solution can be used until the expiration date on the bottle.
-
17 PATIENT COUNSELING INFORMATION
Advise patients to not touch the dropper tip to the eye, eyelids, or any other surface as this may contaminate the solution. Keep the bottle tightly closed.
Instillation Site Pain
Advise patients that drops will sting upon instillation.
Sensitivity to Light and Blurred Vision
Advise patients that they will experience sensitivity to light and blurred vision which may last for a couple of weeks.
Manufactured for:
NorthstarRxLLC
Memphis, TN 38141.
Manufactured by:
Mankind Pharma Limited
Paonta Sahib, Sinnaur
Himachal Pradesh 173025, India.
Revised: September 2025, V-02
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 72603-264-01
Atropine Sulfate Ophthalmic Solution, USP
1%
2 mL
Rx only
Sterile

NDC: 72603-264-01
Atropine Sulfate Ophthalmic Solution, USP
1%
2 mL
Rx only
Sterile

NDC: 72603-264-02
Atropine Sulfate Ophthalmic Solution, USP
1%
5 mL
Rx only
Sterile

NDC: 72603-264-02
Atropine Sulfate Ophthalmic Solution, USP
1%
5 mL
Rx only
Sterile
-
INGREDIENTS AND APPEARANCE
ATROPINE SULFATE
atropine sulfate solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72603-264 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72603-264-01 1 in 1 CARTON 09/01/2024 1 2 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC: 72603-264-02 1 in 1 CARTON 09/01/2024 2 5 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218148 09/01/2024 Labeler - NorthStar RxLLC (830546433) Registrant - Mankind Pharma Limited (915834068) Establishment Name Address ID/FEI Business Operations Mankind Pharma Limited 916512493 MANUFACTURE(72603-264) , ANALYSIS(72603-264) , PACK(72603-264)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.