Cortison Acetate Tablets, USP
CORTISONE ACETATE by
Drug Labeling and Warnings
CORTISONE ACETATE by is a Prescription medication manufactured, distributed, or labeled by Hikma Pharmaceutical. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CORTISONE ACETATE- cortisone acetate tablet
Hikma Pharmaceutical
----------
Cortison Acetate Tablets, USP
DESCRIPTION:
Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract.
Cortisone acetate is a white or practically white, odorless, crystalline powder. It is stable in air. It is insoluble in water. The molecular weight is 402.49. It is designated chemically as 21-(acetyloxy)-17-hydroxypregn-4-ene-3,11,20-trione. The molecular formula is C23H30O6and the structural formula is:
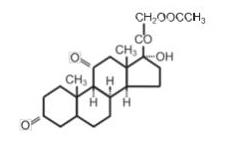
Cortisone AcetateTablets, USP contain 25 mg of cortisone acetate in each tablet.
Inactive ingredients are Anhydrous Lactose, Colloidal Silicon Dioxide, Magnesium Stearate, Microcrystalline Cellulose, Sodium Lauryl Sulfate, and Sodium Starch Glycolate.
CLINICAL PHARMACOLOGY:
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. They are also used for their potent anti-inflammatory effects in disorders of many organ systems.
Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body’s immune responses to diverse stimuli.
INDICATIONS AND USAGE:
1. Endocrine Disorders
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy mineralocorticoid supplementation is of particular importance).
Congenital adrenal hyperplasia Nonsuppurative thyroiditis Hypercalcemia associated with cancer
2. Rheumatic Disorders
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in:
Psoriatic arthritis
Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy)
Ankylosing spondylitis
Acute and subacute bursitis Acute nonspecific tenosynovitis Acute gouty arthritis
Post-traumatic osteoarthritis Synovitis of osteoarthritis Epicondylitis
3. Collagen Diseases
During an exacerbation or as maintenance therapy in selected cases of:
Systemic lupus erythematosus
Acute rheumatic carditis
Systemic dermatomyositis (polymyositis)
4. Dermatologic Diseases
Pemphigus
Bullous dermatitis herpetiformis
Severe erythema multiforme (Stevens-Johnson syndrome) Exfoliative dermatitis
Mycosis fungoides
Severe psoriasis
Severe seborrheic dermatitis
5. Allergic States
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment:
Seasonal or perennial allergic rhinitis
Bronchial asthma Contact dermatitis Atopic dermatitis Serum sickness
Drug hypersensitivity reactions
6. Ophthalmic Diseases
Severe acute and chronic allergic and inflammatory processes
involving the eye and its adnexa, such as: Allergic conjunctivitis
Keratitis
Allergic corneal marginal ulcers Herpes zoster ophthalmicus Iritis and iridocyclitis Chorioretinitis
Anterior segment inflammation
Diffuse posterior uveitis and choroiditis
Optic neuritis
Sympathetic ophthalmia
7. Respiratory Diseases
Symptomatic sarcoidosis
Loeffler’s syndrome not manageable by other means
Berylliosis
Fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculosis chemotherapy
Aspiration pneumonitis
8. Hematologic Disorders
Idiopathic thrombocytopenic purpura in adults Secondary thrombocytopenia in adults Acquired (autoimmune)
hemolytic anemia Erythroblastopenia (RBC anemia)
Congenital (erythroid) hypoplastic anemia
9. Neoplastic Diseases
For palliative management of: Leukemias and lymphomas in adults Acute leukemia of childhood
10. Edematous States
To induce a diuresis or remission of proteinuria in the nephrotic syndrome, without uremia, of the idiopathic type or that due to lupus erythematosus
11. Gastrointestinal Diseases
To tide the patient over a critical period of the disease in: Ulcerative colitis
Regional enteritis
12. Miscellaneous
Tuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate antituberculous chemotherapy
Trichinosis with neurologic or myocardial involvement
WARNINGS:
In patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is indicated.
Drug-induced secondary adrenocortical insufficiency may result from too rapid withdrawal of corticosteroids and may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. If the patient is receiving steroids already, dosage may have to be increased. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
Corticosteroids may mask some signs of infection, and new infections may appear during their use. There may be decreased resistance and inability to localize infection when corticosteroids are used. Moreover, corticosteroids may affect the nitroblue-tetrazolium test for bacterial infection and produce false negative results.
In cerebral malaria, a double-blind trial has shown that the use of corticosteroids is associated with prolongation of coma and a higher incidence of pneumonia and gastrointestinal bleeding.
Corticosteroids may activate latent amebiasis. Therefore, it is recommended that latent or active amebiasis be ruled out before initiating corticosteroid therapy in any patient who has time in the tropics or any patient with unexplained diarrhea.
Prolonged use of corticosteroids may produce posterior subsapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to fungi or viruses.
Average and large doses of hydrocortisone or cortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Administration of live virus vaccines, including smallpox, is contraindicated in individuals receiving immunosuppressive doses of corticosteroids. If inactivated viral or bacterial vaccines are administered to individuals receiving immunosuppressive doses of corticosteroids, the expected serum antibody response may not be obtained. However, immunization procedures may be undertaken in patients who are receiving corticosteroids as replacement therapy, e.g., for Addison’s disease.
Persons who are on drugs which suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have more serious or even fatal course in non-immune children or adults on corticosteroids. In such children or adults who have not had these diseases, particular care should be taken to avoid exposure. How the dose, route and duration of corticosteroid administration affects the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information). If chickenpox develops, treatment with antiviral agents may be considered.
The use of cortisone acetate tablets in active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate antituberculous regimen.
If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
Usage in pregnancy: Since adequate human reproduction studies have not been done with corticosteroids, use of these drugs in pregnancy or in women of childbearing potential requires that the anticipated benefits be weighed against the possible hazards to the mother and embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Corticosteroids appear in breast milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other unwanted effects. Mothers taking pharmacologic doses of corticosteroids should be advised not to nurse.
PRECAUTIONS:
General:
Following prolonged therapy, withdrawal of corticosteroids may result in symptoms of the corticosteroid withdrawal syndrome including fever, myalgia, arthralgia, and malaise. This may occur in patients even without evidence of adrenal insufficiency.
There is an enhanced effect of corticosteroids in patients with hypothyroidism and in those with cirrhosis.
Corticosteroids should be used cautiously in patients with ocular herpes simplex because of possible corneal perforation.
The lowest possible dose of corticosteroid should be used to control the condition under treatment, and when reduction in dosage is possible, the reduction should be gradual.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Aspirin should be used cautiously in conjunction with corticosteroids in hypoprothrombinemia.
Steroids should be used with caution in nonspecific ulcerative colitis, if there is a probability of impending perforation, abscess, or other pyogenic infection, diverticulitis, fresh intestinal anastomoses, active or latent peptic ulcer, renal insufficiency, hypertension, osteoporosis, and myasthenia gravis. Signs of peritoneal irritation following gastrointestinal perforation in patients receiving large doses of corticosteroids may be minimal or absent. Fat embolism has been reported as a possible complication of hypercortisonism.
When large doses are given, some authorities advise that corticosteroids be taken with meals and antacids taken between meals to help to prevent peptic ulcer.
Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed.
Steroids may increase or decrease motility and number of spermatozoa in some patients.
Phenytoin, phenobarbital, ephedrine, and rifampin may enhance the metabolic clearance of corticosteroids, resulting in decreased blood levels and lessened physiologic activity, thus requiring adjustment in corticosteroid dosage.
The prothrombin time should be checked frequently in patients who are receiving corticosteroids and coumarin anticoagulants at the same time because of reports that corticosteroids have altered the response to these anticoagulants. Studies have shown that the usual effect produced by adding corticosteroids is inhibition of response to coumarins, although there have been some conflicting reports of potentiation not substantiated by studies.
When corticosteroids are administered concomitantly with potassium-depleting diuretics, patients should be observed closely for development of hypokalemia.
ADVERSE REACTIONS:
Fluid and Electrolyte Disturbances
Sodium retention
Fluid retention
Congestive heart failure in susceptible patients
Potassium loss Hypokalemic alkalosis Hypertension
Musculoskeletal Muscle weakness Steroid myopathy Loss of muscle mass Osteoporosis
Vertebral compression fractures
Aseptic necrosis of femoral and humeral heads
Pathologic fracture of long bones
Tendon rupture
Gastrointestinal
Peptic ulcer with possible perforation and hemorrhage Perforation of the small and large bowel, particularly in patients with
inflammatory bowel disease
Pancreatitis Abdominal distention Ulcerative esophagitis
Dermatologic
Impaired wound healing
Thin fragile skin
Petechiae and ecchymoses
Erythema
Increased sweating
May suppress reactions to skin tests
Other cutaneous reactions, such as allergic dermatitis, urticaria, angioneurotic edema
Neurologic
Convulsions
Increased intracranial pressure with papilledema (pseudotumor cerbri) usually after treatment
Vertigo
Headache
Psychic disturbances
Endocrine
Menstrual irregularities Development of cushingoid state Suppression of growth in children
Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma,
surgery, or illness Decreased carbohydrate tolerance
Manifestations of latent diabetes mellitus
Increased requirements for insulin or oral hypoglycemic agents in diabetics
Hirsutism
Ophthalmic
Posterior subcapsular cataracts Increased intraocular pressure Glaucoma
Exophthalmos
Metabolic
Negative nitrogen balance due to protein catabolism
Cardiovascular
Myocardial rupture following recent myocardial infarctions (see WARNINGS).
Other Hypersensitivity Thromboembolism Weight gain Increased appetite Nausea
Malaise
OVERDOSAGE:
Reports of acute toxicity and/or death following overdosage of glucocorticoids are rare. In the event of overdosage, no specific antidote is available; treatment is supportive and symptomatic.
The intraperitoneal LD50 of cortisone acetate in female mice was 1405 mg/kg.
DOSAGE AND ADMINISTRATION:
For Oral Administration
DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE AND THE RESPONSE OF THE PATIENT.
The initial dosage varies from 25 to 300 mg a day depending on the disease being treated. In less severe diseases doses lower than
25 mg may suffice, while in severe diseases doses higher than 300 mg may be required. The initial dosage should be maintained or adjusted until the patient’s response is satisfactory. If satisfactory clinical response does not occur after a reasonable period of time, discontinue cortisone acetate tablets and transfer the patient to other therapy.
After a favorable initial response, the proper maintenance dosage should be determined by decreasing the initial dosage in small amounts to the lowest dosage that maintains an adequate clinical response.
Patients should be observed closely for signs that might require dosage adjustment, including changes in clinical status resulting from remissions or exacerbations of the disease, individual drug responsiveness, and the effect of stress (e.g., surgery, infection, trauma). During stress it may be necessary to increase dosage temporarily.
If the drug is to be stopped after more than a few days of treatment, it usually should be withdrawn gradually.
HOW SUPPLIED:
Cortisone Acetate Tablets, USP 25 mg: White, Round, Scored Tablet; Imprinted “West-ward 202.”
Bottles of 100 tablets.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Manufactured by: HIKMA Pharmaceuticals P.O. Box 182400
Amman 11118 – Jordan
Distributed by:
West-Ward Pharmaceutical Corp.
Eatontown, NJ 07724 USA
Issued September 2013
Principal Display Panel
NDC: 59115-161-01
Cortison Acetate
Tablets, USP
25 mg
Rx Only
100 Tablets
Principal Display Panel
NDC: 59115-161-99
BULK SHIPMENT
Cortison Acetate Tablets USP,
25 mg

| CORTISONE ACETATE
cortisone acetate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hikma Pharmaceutical (534661645) |