Acutens - Cool & Heat (80551-002) Sore Muscle Roller
Cool and Heat Sore Muscle Roller by
Drug Labeling and Warnings
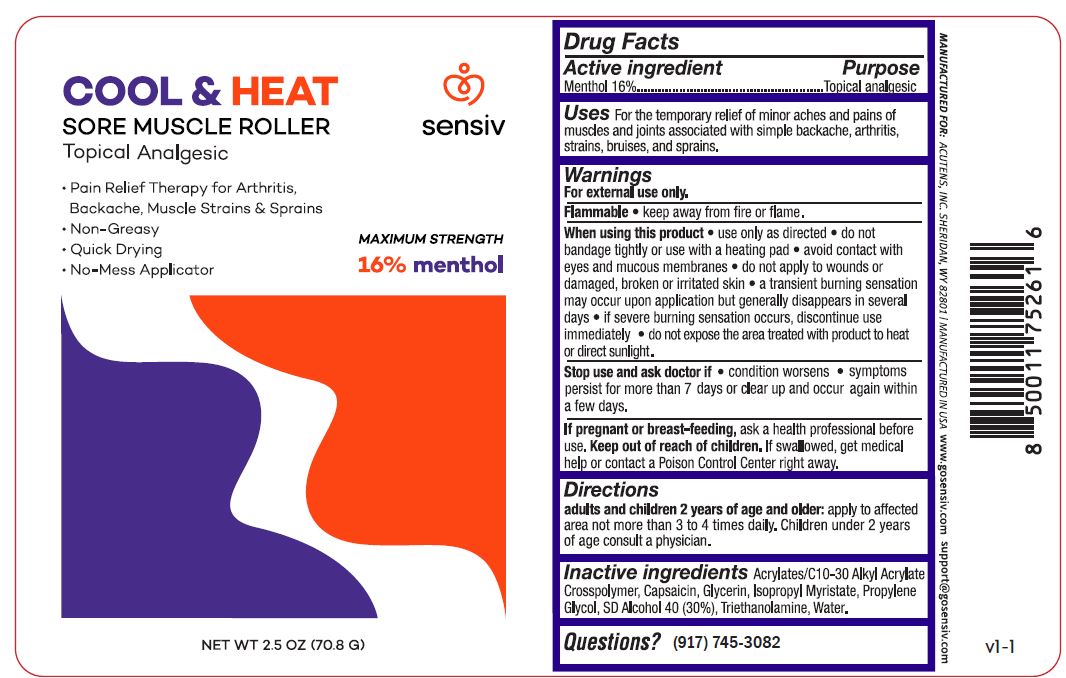
Cool and Heat Sore Muscle Roller by is a Otc medication manufactured, distributed, or labeled by Acutens, Inspec Solutions LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COOL AND HEAT SORE MUSCLE ROLLER- menthol gel
Acutens
----------
Acutens - Cool & Heat (80551-002)
Sore Muscle Roller
Uses:
Temporary relief of minor aches pains of muscles and joints associated with arthritis, simple backache, strains, bruises, and sprains
Warnings
For external use only
Flammable: Keep away from fire or flame.
When using this product use only as directed do not bandage tightly or use with a heating pad avoid contact with eyes and mucous membranes do not apply to wounds or damaged, broken or irritated skin a transient burning sensation may occur upon application but generally disappears in several days if severe burning sensation occurs, discontinue use immediately do not expose the area treated with product to heat or direct sunlight.
Stop use and ask doctor if condition worsens symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.If accidentally ingested get medical help or contact a Poison Control Center immediately
Direction
Adults and children 2 years of age and older: apply to affected area not more than 3-4 times daily
Children under 2 years old: consult apfysician
| COOL AND HEAT SORE MUSCLE ROLLER
menthol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Acutens (051133165) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Inspec Solutions | 081030372 | manufacture(80551-002) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.