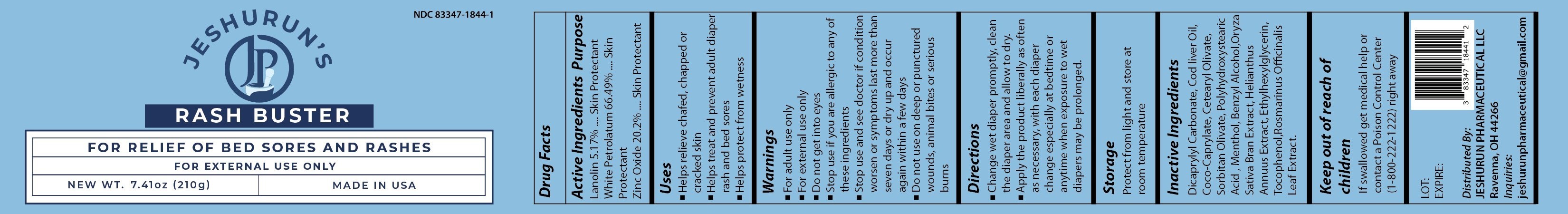
RASH BUSTER- zinc oxide paste paste
Rash Buster by
Drug Labeling and Warnings
Rash Buster by is a Otc medication manufactured, distributed, or labeled by Jeshurun Pharmaceutical LLC, Chae Organics, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Inactive ingredients
- Dosage and adminstration
- Precaution section
- Adverse reactions
- Indication & usage
- OTC- Active ingredient
- Health claim
- Purpose
- Ask doctor
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- LABEL AND DRUG FACTS
-
INGREDIENTS AND APPEARANCE
RASH BUSTER
zinc oxide paste pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 83347-1844 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 42.42 g in 210 g WHITE PETROLATUM (UNII: B6E5W8RQJ4) (WHITE PETROLATUM - UNII:B6E5W8RQJ4) WHITE PETROLATUM 139.629 g in 210 g LANOLIN (UNII: 7EV65EAW6H) (LANOLIN - UNII:7EV65EAW6H) LANOLIN 10.857 g in 210 g Inactive Ingredients Ingredient Name Strength COD LIVER OIL (UNII: BBL281NWFG) Product Characteristics Color white (White Paste) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83347-1844-2 24 in 1 BOX 05/01/2023 1 NDC: 83347-1844-1 210 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/01/2023 Labeler - Jeshurun Pharmaceutical LLC (092600853) Registrant - Jeshurun Pharmaceutical LLC (092600853) Establishment Name Address ID/FEI Business Operations Chae Organics, LLC 828138375 manufacture(83347-1844)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.