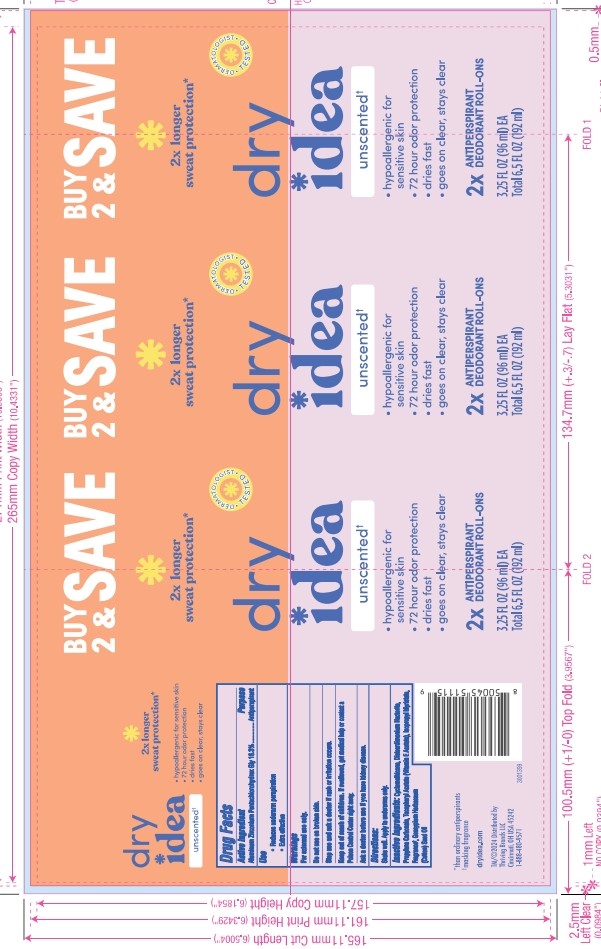
DRY IDEA ADVANCED DRY ROLL-ON ANTIPERSPIRANT AND DEODORANT UNSCENTED liquid
Dry Idea Advanced Dry Roll-On Antiperspirant and Deodorant Unscented by
Drug Labeling and Warnings
Dry Idea Advanced Dry Roll-On Antiperspirant and Deodorant Unscented by is a Otc medication manufactured, distributed, or labeled by Thriving Brands LLC, KDC/One Development Corporation, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DOSAGE & ADMINISTRATION
- ACTIVE INGREDIENT
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- STOP USE
- INDICATIONS & USAGE
- WARNINGS
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DRY IDEA ADVANCED DRY ROLL-ON ANTIPERSPIRANT AND DEODORANT UNSCENTED
dry idea advanced dry roll-on antiperspirant and deodorant unscented liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82699-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM PENTACHLOROHYDREX GLY (UNII: 94703016SM) (ALUMINUM ZIRCONIUM PENTACHLOROHYDREX GLY - UNII:94703016SM) ALUMINUM ZIRCONIUM PENTACHLOROHYDREX GLY 16.3 g in 96 mL Inactive Ingredients Ingredient Name Strength DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) Product Characteristics Color brown (Light) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82699-202-03 96 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 07/03/2023 10/24/2024 2 NDC: 82699-202-04 192 mL in 1 PACKAGE; Type 0: Not a Combination Product 07/03/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 07/03/2023 Labeler - Thriving Brands LLC (118346160)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.