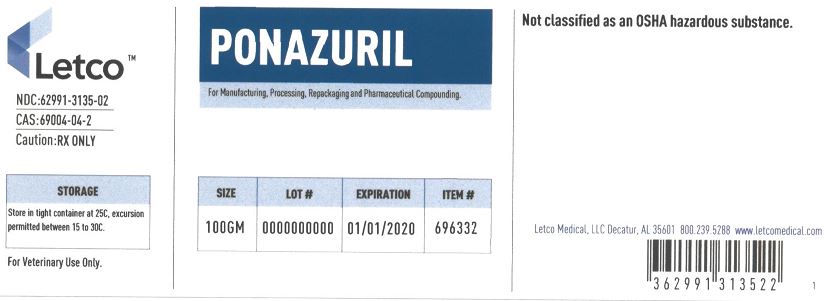
Ponazuril by LETCO MEDICAL, LLC PONAZURIL powder
Ponazuril by
Drug Labeling and Warnings
Ponazuril by is a Other medication manufactured, distributed, or labeled by LETCO MEDICAL, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PONAZURIL
ponazuril powderProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC: 62991-3135 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONAZURIL (UNII: JPW84AS66U) (PONAZURIL - UNII:JPW84AS66U) PONAZURIL 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62991-3135-1 25 g in 1 JAR 09/24/2018 2 NDC: 62991-3135-2 100 g in 1 JAR 09/24/2018 3 NDC: 62991-3135-3 500 g in 1 JAR 08/19/2019 02/28/2021 4 NDC: 62991-3135-4 1000 g in 1 JAR 08/19/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BULK INGREDIENT FOR ANIMAL DRUG COMPOUNDING 09/24/2018 Labeler - LETCO MEDICAL, LLC (079891426)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.