CEFUROXIME AXETIL tablet
cefuroxime axetil by
Drug Labeling and Warnings
cefuroxime axetil by is a Prescription medication manufactured, distributed, or labeled by Lupin Pharmaceuticals, Inc., LUPIN LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CEFUROXIME AXETIL TABLETS safely and effectively. See full prescribing information for CEFUROXIME AXETIL TABLETS.
CEFUROXIME AXETIL tablets, for oral use
Initial U.S. Approval: 1987INDICATIONS AND USAGE
Cefuroxime axetil tablets are a cephalosporin antibacterial drug indicated for the treatment of the following infections due to susceptible bacteria: (1)
- Pharyngitis/tonsillitis (adults and pediatric patients) (1.1)
- Acute bacterial otitis media (pediatric patients) (1.2)
- Acute bacterial maxillary sinusitis (adults and pediatric patients) (1.3)
- Acute bacterial exacerbations of chronic bronchitis (adults and pediatric patients 13 years and older) (1.4)
- Uncomplicated skin and skin-structure infections (adults and pediatric patients 13 years and older) (1.5)
- Uncomplicated urinary tract infections (adults and pediatric patients 13 years and older) (1.6)
- Uncomplicated gonorrhea (adults and pediatric patients 13 years and older) (1.7)
- Early Lyme disease (adults and pediatric patients 13 years and older) (1.8)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefuroxime axetil tablets and other antibacterial drugs, cefuroxime axetil tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DOSAGE AND ADMINISTRATION
- Tablets and oral suspension are not bioequivalent and are therefore not substitutable on a milligram-per-milligram basis. (2.1)
- Administer tablets with or without food. (2.2)
- Administer cefuroxime axetil tablets as described in the dosage guidelines. (2.2)
- Dosage adjustment is required for patients with impaired renal function. (2.5)
Adult Patients and Pediatric Patients Dosage Guidelines for Cefuroxime Axetil Tablets Infection
Dosage
Duration (Days)
Adults and Adolescents (13 years and older)
Pharyngitis/tonsillitis (mild to moderate)
250 mg every 12 hours
10
Acute bacterial maxillary sinusitis (mild to moderate)
250 mg every 12 hours
10
Acute bacterial exacerbations of chronic bronchitis (mild to moderate)
250 or 500 mg every 12 hours
10
Uncomplicated skin and skin-structure infections
250 or 500 mg every 12 hours
10
Uncomplicated urinary tract infections
250 mg every 12 hours
7 to 10
Uncomplicated gonorrhea
1,000 mg
single dose
Early Lyme disease
500 mg every 12 hours
20
Pediatric Patients younger than 13 years (who can swallow tablets whole)
Acute bacterial otitis media
250 mg every 12 hours
Acute bacterial maxillary sinusitis
250 mg every 12 hours
DOSAGE FORMS AND STRENGTHS
- Tablets: 250 mg and 500 mg (3)
CONTRAINDICATIONS
Known hypersensitivity (e.g., anaphylaxis) to cefuroxime axetil tablets or to other β-lactams (e.g., penicillins and cephalosporins). (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (≥3%) for cefuroxime axetil tablets are diarrhea, nausea/vomiting, Jarisch-Herxheimer reaction, and vaginitis (early Lyme disease). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals USA Inc. at 1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pharyngitis/Tonsillitis
1.2 Acute Bacterial Otitis Media
1.3 Acute Bacterial Maxillary Sinusitis
1.4 Acute Bacterial Exacerbations of Chronic Bronchitis
1.5 Uncomplicated Skin and Skin-Structure Infections
1.6 Uncomplicated Urinary Tract Infections
1.7 Uncomplicated Gonorrhea
1.8 Early Lyme Disease (erythema migrans)
1.10 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage for Cefuroxime Axetil Tablets
2.5 Dosage in Patients with Impaired Renal Function
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylactic Reactions
5.2 Clostridium difficile-Associated Diarrhea
5.3 Potential for Microbial Overgrowth
5.4 Development of Drug-Resistant Bacteria
5.6 Interference with Glucose Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs that Reduce Gastric Acidity
7.2 Probenecid
7.3 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Acute Bacterial Maxillary Sinusitis
14.2 Early Lyme Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pharyngitis/Tonsillitis
Cefuroxime axetil tablets are indicated for the treatment of adult patients and pediatric patients (13 years and older) with mild-to-moderate pharyngitis/tonsillitis caused by susceptible strains of Streptococcus pyogenes.
Limitations of Use
- The efficacy of cefuroxime axetil in the prevention of rheumatic fever was not established in clinical trials.
- The efficacy of cefuroxime axetil in the treatment of penicillin-resistant strains of Streptococcus pyogenes has not been demonstrated in clinical trials.
1.2 Acute Bacterial Otitis Media
Cefuroxime axetil tablets are indicated for the treatment of pediatric patients (who can swallow tablets whole) with acute bacterial otitis media caused by susceptible strains of Streptococcus pneumoniae, Haemophilus influenzae (including β-lactamase–producing strains), Moraxella catarrhalis (including β-lactamase–producing strains), or Streptococcus pyogenes.
1.3 Acute Bacterial Maxillary Sinusitis
Cefuroxime axetil tablets are indicated for the treatment of adult and pediatric patients (13 years and older) with mild-to-moderate acute bacterial maxillary sinusitis caused by susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae (non-β-lactamase–producing strains only).
Limitations of Use
The effectiveness of cefuroxime axetil for sinus infections caused by β-lactamase–producing Haemophilus influenzae or Moraxella catarrhalis in patients with acute bacterial maxillary sinusitis was not established due to insufficient numbers of these isolates in the clinical trials [see CLINICAL STUDIES (14.1)].
1.4 Acute Bacterial Exacerbations of Chronic Bronchitis
Cefuroxime axetil tablets are indicated for the treatment of adult patients and pediatric patients (aged 13 and older) with mild-to-moderate acute bacterial exacerbations of chronic bronchitis caused by susceptible strains of Streptococcus pneumoniae, Haemophilus influenzae (β-lactamase–negative strains), or Haemophilus parainfluenzae (β-lactamase–negative strains).
1.5 Uncomplicated Skin and Skin-Structure Infections
Cefuroxime axetil tablets are indicated for the treatment of adult patients and pediatric patients (aged 13 and older) with uncomplicated skin and skin-structure infections caused by susceptible strains of Staphylococcus aureus (including β-lactamase–producing strains) or Streptococcus pyogenes.
1.6 Uncomplicated Urinary Tract Infections
Cefuroxime axetil tablets are indicated for the treatment of adult patients and pediatric patients (aged 13 and older) with uncomplicated urinary tract infections caused by susceptible strains of Escherichia coli or Klebsiella pneumoniae.
1.7 Uncomplicated Gonorrhea
Cefuroxime axetil tablets are indicated for the treatment of adult patients and pediatric patients (aged 13 and older) with uncomplicated gonorrhea, urethral and endocervical, caused by penicillinase producing and non-penicillinase–producing susceptible strains of Neisseria gonorrhoeae and uncomplicated gonorrhea, rectal, in females, caused by non-penicillinase–producing susceptible strains of Neisseria gonorrhoeae.
1.8 Early Lyme Disease (erythema migrans)
Cefuroxime axetil tablets are indicated for the treatment of adult patients and pediatric patients (aged 13 and older) with early Lyme disease (erythema migrans) caused by susceptible strains of Borrelia burgdorferi.
1.10 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefuroxime axetil and other antibacterial drugs, cefuroxime axetil should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Cefuroxime axetil tablets and cefuroxime axetil for oral suspension are not bioequivalent and are therefore not substitutable on a milligram-per-milligram basis [see CLINICAL PHARMACOLOGY (12.3)] .
- Administer cefuroxime axetil tablets as described in the appropriate dosage guidelines [see DOSAGE AND ADMINISTRATION (2.2)].
- Administer cefuroxime axetil tablets with or without food.
- Pediatric patients (aged 13 years and older) who cannot swallow the cefuroxime axetil tablets whole should receive cefuroxime axetil for oral suspension because the tablet has a strong, persistent bitter taste when crushed [see DOSAGE AND ADMINISTRATION (2.2)].
2.2 Dosage for Cefuroxime Axetil Tablets
Administer cefuroxime axetil tablets as described in the dosage guidelines table below with or without food.
Table 1. Adult Patients and Pediatric Patients Dosage Guidelines for Cefuroxime Axetil Tablets - * The safety and effectiveness of cefuroxime axetil administered for less than 10 days in patients with acute exacerbations of chronic bronchitis have not been established.
- † When crushed, the tablet has a strong, persistent bitter taste. Therefore, patients who cannot swallow the tablet whole should receive the oral suspension.
Infection
Dosage
Duration (Days)
Adults and Adolescents (13 years and older)
Pharyngitis/tonsillitis (mild to moderate)
250 mg every 12 hours
10
Acute bacterial maxillary sinusitis (mild to moderate)
250 mg every 12 hours
10
Acute bacterial exacerbations of chronic bronchitis (mild to moderate)
250 or 500 mg every 12 hours
10*
Uncomplicated skin and skin-structure infections
250 or 500 mg every 12 hours
10
Uncomplicated urinary tract infections
250 mg every 12 hours
7 to 10
Uncomplicated gonorrhea
1,000 mg
single dose
Early Lyme disease
500 mg every 12 hours
20
Pediatric Patients younger than 13 years (who can swallow tablets whole)†
Acute bacterial otitis media
250 mg every 12 hours
10
Acute bacterial maxillary sinusitis
250 mg every 12 hours
10
2.5 Dosage in Patients with Impaired Renal Function
A dosage interval adjustment is required for patients whose creatinine clearance is less than 30 mL/min, as listed in Table 4 below, because cefuroxime is eliminated primarily by the kidney [see CLINICAL PHARMACOLOGY (12.3)].
Table 4. Dosing in Adults with Renal Impairment Creatinine Clearance (mL/min)
Recommended Dosage
≥30
No dosage adjustment
10 to ˂30
Standard individual dose given every 24 hours
˂10 (without hemodialysis)
Standard individual dose given every 48 hours
Hemodialysis
A single additional standard dose should be given at the end of each dialysis
-
3 DOSAGE FORMS AND STRENGTHS
Cefuroxime axetil tablets USP are off-white, capsule-shaped, film-coated tablets available in the following strengths:
- 250 mg of cefuroxime (as cefuroxime axetil) are white to off-white, capsule-shaped, film-coated tablets with "LUPIN" debossed on one side and "302" on the other side.
- 500 mg of cefuroxime (as cefuroxime axetil) are white to off-white, capsule-shaped, film-coated tablets with "LUPIN" debossed on one side and "303" on the other side.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylactic Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on β-lactam antibacterials, including cefuroxime axetil [see ADVERSE REACTIONS (6.2)]. These reactions are more likely to occur in individuals with a history of β-lactam hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins. Cefuroxime axetil is contraindicated in patients with a known hypersensitivity to cefuroxime axetil or other β-lactam antibacterial drugs [see CONTRAINDICATIONS (4)]. Before initiating therapy with cefuroxime axetil, inquire about previous hypersensitivity reactions to penicillins, cephalosporins, or other allergens. If an allergic reaction occurs, discontinue cefuroxime axetil and institute appropriate therapy.
5.2 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefuroxime axetil, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Potential for Microbial Overgrowth
The possibility of superinfections with fungal or bacterial pathogens should be considered during therapy.
5.4 Development of Drug-Resistant Bacteria
Prescribing cefuroxime axetil either in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.6 Interference with Glucose Tests
A false-positive result for glucose in the urine may occur with copper reduction tests, and a false-negative result for blood/plasma glucose may occur with ferricyanide tests in subjects receiving cefuroxime axetil [see DRUG INTERACTIONS (7.3)].
-
6 ADVERSE REACTIONS
The following serious and otherwise important adverse reaction is described in greater detail in the Warnings and Precautions section of the label:
Anaphylactic Reactions [see WARNINGS AND PRECAUTIONS (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Tablets
Multiple-Dose Dosing Regimens with 7 to 10 Days' Duration: In multiple-dose clinical trials, 912 subjects were treated with cefuroxime axetil (125 to 500 mg twice daily). It is noted that 125 mg twice daily is not an approved dosage. Twenty (2.2%) subjects discontinued medication due to adverse reactions. Seventeen (85%) of the 20 subjects who discontinued therapy did so because of gastrointestinal disturbances, including diarrhea, nausea, vomiting, and abdominal pain. The percentage of subjects treated with cefuroxime axetil who discontinued study drug because of adverse reactions was similar at daily doses of 1,000, 500, and 250 mg (2.3%, 2.1%, and 2.2%, respectively). However, the incidence of gastrointestinal adverse reactions increased with the higher recommended doses.
The adverse reactions in Table 5 are for subjects (n = 912) treated with cefuroxime axetil in multiple-dose clinical trials.
Table 5. Adverse Reactions (≥1%) after Multiple-Dose Regimens with Cefuroxime Axetil Tablets Adverse Reaction
Cefuroxime Axetil Tablets (n = 912)
Blood and lymphatic system disorders
Eosinophilia
1%
Gastrointestinal disorders
Diarrhea
4%
Nausea/Vomiting
3%
Investigations
Transient elevation in AST
2%
Transient elevation in ALT
2%
Transient elevation in LDH
1%
The following adverse reactions occurred in less than 1% but greater than 0.1% of subjects (n = 912) treated with cefuroxime axetil in multiple-dose clinical trials.
Immune System Disorders: Hives, swollen tongue.
Metabolism and Nutrition Disorders: Anorexia.
Nervous System Disorders: Headache.
Cardiac Disorders: Chest pain.
Respiratory Disorders: Shortness of breath.
Gastrointestinal Disorders: Abdominal pain, abdominal cramps, flatulence, indigestion, mouth ulcers.
Skin and Subcutaneous Tissue Disorders: Rash, itch
Renal and Urinary Disorders: Dysuria.
Reproductive System and Breast Disorders: Vaginitis, vulvar itch.
General Disorders and Administration Site Conditions: Chills, sleepiness, thirst.
Investigations: Positive Coombs' test.
Early Lyme Disease with 20-Day Regimen: Two multicenter trials assessed cefuroxime axetil tablets 500 mg twice daily for 20 days. The most common drug-related adverse experiences were diarrhea (10.6%), Jarisch-Herxheimer reaction (5.6%), and vaginitis (5.4%). Other adverse experiences occurred with frequencies comparable to those reported with 7 to 10 days' dosing.
Single-Dose Regimen for Uncomplicated Gonorrhea: In clinical trials using a single 1,000-mg dose of cefuroxime axetil tablets, 1,061 subjects were treated for uncomplicated gonorrhea.
The adverse reactions in Table 6 were for subjects treated with a single dose of 1,000 mg cefuroxime axetil tablets in U.S. clinical trials.
Table 6. Adverse Reactions (≥1%) after Single-Dose Regimen with 1,000-mg Cefuroxime Axetil Tablets for Uncomplicated Gonorrhea Adverse Reaction
Cefuroxime Axetil Tablets
(n = 1,061)
Gastrointestinal disorders
Nausea/Vomiting
7%
Diarrhea
4%
The following adverse reactions occurred in less than 1% but greater than 0.1% of subjects (n = 1,061) treated with a single dose of cefuroxime axetil tablets 1,000 mg for uncomplicated gonorrhea in U.S. clinical trials.
Infections and Infestations: Vaginal candidiasis.
Nervous System Disorders: Headache, dizziness, somnolence.
Cardiac Disorders: Tightness/pain in chest, tachycardia.
Gastrointestinal Disorders: Abdominal pain, dyspepsia.
Skin and Subcutaneous Tissue Disorders: Erythema, rash, pruritus.
Musculoskeletal and Connective Tissue Disorders: Muscle cramps, muscle stiffness, muscle spasm of neck, lockjaw-type reaction.
Renal and Urinary Disorders: Bleeding/pain in urethra, kidney pain.
Reproductive System and Breast Disorders: Vaginal itch, vaginal discharge.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of cefuroxime axetil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders
Hemolytic anemia, leukopenia, pancytopenia, thrombocytopenia.
Gastrointestinal Disorders
Pseudomembranous colitis [see WARNINGS AND PRECAUTIONS (5.2)].
Hepatobiliary Disorders
Hepatic impairment including hepatitis and cholestasis, jaundice.
Immune System Disorders
Anaphylaxis, serum sickness-like reaction.
Investigations
Increased prothrombin time.
Nervous System Disorders
Seizure, encephalopathy.
Renal and Urinary Disorders
Renal dysfunction.
Skin and Subcutaneous Tissue Disorders
Angioedema, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria.
-
7 DRUG INTERACTIONS
7.1 Drugs that Reduce Gastric Acidity
Drugs that reduce gastric acidity may result in a lower bioavailability of cefuroxime axetil compared with administration in the fasting state. Administration of drugs that reduce gastric acidity may negate the food effect of increased absorption of cefuroxime axetil when administered in the postprandial state. Administer cefuroxime axetil at least 1 hour before or 2 hours after administration of short-acting antacids. Histamine-2 (H2) antagonists and proton pump inhibitors should be avoided.
7.2 Probenecid
Concomitant administration of probenecid with cefuroxime axetil tablets increases serum concentrations of cefuroxime [see CLINICAL PHARMACOLOGY (12.3)]. Coadministration of probenecid with cefuroxime axetil is not recommended.
7.3 Drug/Laboratory Test Interactions
A false-positive reaction for glucose in the urine may occur with copper reduction tests (e.g., Benedict's or Fehling's solution), but not with enzyme-based tests for glycosuria. As a false-negative result may occur in the ferricyanide test, it is recommended that either the glucose oxidase or hexokinase method be used to determine blood/plasma glucose levels in patients receiving cefuroxime axetil. The presence of cefuroxime does not interfere with the assay of serum and urine creatinine by the alkaline picrate method.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Available data from published epidemiologic studies, case series, and case reports over several decades with cephalosporin use, including cefuroxime axetil, in pregnant women have not established drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data).
In studies in pregnant mice and rats administered oral cefuroxime axetil during organogenesis at 14 and 9 times the maximum recommended human dose (MRHD) based on body surface area, respectively, there were no adverse developmental outcomes (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: Maternal gonorrhea may be associated with preterm birth, low neonatal birth weight, chorioamnionitis, intrauterine growth restriction, small for gestational age, and premature rupture of membranes. Perinatal transmission of gonorrhea to the offspring can result in infant blindness, joint infections, and bloodstream infections.
Data
Human Data: While available studies cannot definitively establish the absence of risk, published data from epidemiologic studies, case series, and case reports over several decades have not identified an association with cephalosporin use (including cefuroxime axetil) during pregnancy and major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Available studies have methodologic limitations, including small sample size, retrospective data collection, and inconsistent comparator groups.
Animal Data: Studies performed with oral cefuroxime axetil administered to pregnant mice during organogenesis (Gestation Days 7 through 16) at doses up to 3,200 mg/kg/day (14 times the MRHD based on body surface area); and in rats dosed during organogenesis and lactation (Gestation Days 7 through 16 and Gestation Days 17 through Lactation Day 21, respectively) at doses up to 1,000 mg/kg/day (9 times the MRHD based on body surface area) have revealed no adverse developmental outcomes.
8.2 Lactation
Based on several published case reports describing multiple lactating women who received cefuroxime via intravenous, intramuscular, and oral routes, cefuroxime is present in human milk. The highest maternal milk concentration described occurred in lactating women 8 hours after an intramuscular administration of cefuroxime 750 mg. Allowing for an infant milk consumption of 150 mL/kg/day, the estimated breastfed infant dose would be less than 1% of the adult dose. No data are available on the effects of the drug on the breastfed infant or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for cefuroxime and any potential adverse effects on the breastfed infant from cefuroxime or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of cefuroxime axetil have been established for pediatric patients aged 3 months to 12 years for acute bacterial maxillary sinusitis based upon its approval in adults. Use of cefuroxime axetil in pediatric patients is supported by pharmacokinetic and safety data in adults and pediatric patients, and by clinical and microbiological data from adequate and well-controlled trials of the treatment of acute bacterial maxillary sinusitis in adults and of acute otitis media with effusion in pediatric patients. It is also supported by postmarketing adverse events surveillance. [See INDICATIONS AND USAGE (1), DOSAGE AND ADMINISTRATION (2), ADVERSE REACTIONS (6), CLINICAL PHARMACOLOGY (12.3)].
8.5 Geriatric Use
Of the total number of subjects who received cefuroxime axetil in 20 clinical trials, 375 were aged 65 and older while 151 were aged 75 and older. No overall differences in safety or effectiveness were observed between these subjects and younger adult subjects. Reported clinical experience has not identified differences in responses between the elderly and younger adult patients, but greater sensitivity of some older individuals cannot be ruled out.
Cefuroxime is substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Cefuroxime axetil tablets USP contain cefuroxime as cefuroxime axetil. Cefuroxime axetil is a semisynthetic, cephalosporin antibacterial drug for oral administration.
The chemical name of cefuroxime axetil (1-(acetyloxy) ethyl ester of cefuroxime) is (RS)-1-hydroxyethyl (6R,7R)-7-[2-(2-furyl)glyoxyl-amido]-3-(hydroxymethyl)-8-oxo-5-thia-1- azabicyclo[4.2.0]-oct-2-ene-2-carboxylate, 72-(Z)-(O-methyl-oxime), 1-acetate 3-carbamate. Its molecular formula is C20H22N4O10S, and it has a molecular weight of 510.48.
Cefuroxime axetil is in the amorphous form and has the following structural formula:
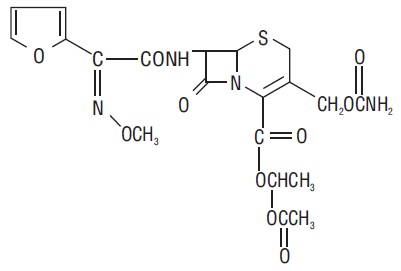
Cefuroxime axetil tablets USP are film-coated and contain the equivalent of 250 or 500 mg of cefuroxime as cefuroxime axetil. Cefuroxime axetil tablets USP contain the inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydrogenated vegetable oil, hypromellose, microcrystalline cellulose, propylene glycol, polyethylene glycol, sodium lauryl sulfate, talc and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
After oral administration, cefuroxime axetil is absorbed from the gastrointestinal tract and rapidly hydrolyzed by nonspecific esterases in the intestinal mucosa and blood to cefuroxime. Serum pharmacokinetic parameters for cefuroxime following administration of cefuroxime axetil tablets to adults are shown in Table 8.
Table 8. Pharmacokinetics of Cefuroxime Administered in the Postprandial State as Cefuroxime Axetil Tablets to Adults* - * Mean values of 12 healthy adult volunteers.
- † Drug administered immediately after a meal.
Dose† (Cefuroxime
Equivalent)
Peak Plasma Concentration
(mcg/mL)
Time of Peak Plasma Concentration
(h)
Mean Elimination
Half-life (h)
AUC
(mcg●h/mL)
125 mg
250 mg
500 mg
1,000 mg
2.1
4.1
7.0
13.6
2.2
2.5
3.0
2.5
1.2
1.2
1.2
1.3
6.7
12.9
27.4
50.0
Effect of Food: Absorption of the tablet is greater when taken after food (absolute bioavailability increases from 37% to 52%). Despite this difference in absorption, the clinical and bacteriologic responses of subjects were independent of food intake at the time of tablet administration in 2 trials where this was assessed.
All pharmacokinetic and clinical effectiveness and safety trials in pediatric subjects using the suspension formulation were conducted in the fed state. No data are available on the absorption kinetics of the suspension formulation when administered to fasted pediatric subjects.
Lack of Bioequivalence: Oral suspension was not bioequivalent to tablets when tested in healthy adults. The tablet and oral suspension formulations are NOT substitutable on a milligram-per-milligram basis. The area under the curve for the suspension averaged 91% of that for the tablet, and the peak plasma concentration for the suspension averaged 71% of the peak plasma concentration of the tablets. Therefore, the safety and effectiveness of both the tablet and oral suspension formulations were established in separate clinical trials.
Distribution
Cefuroxime is distributed throughout the extracellular fluids. Approximately 50% of serum cefuroxime is bound to protein.
Metabolism
The axetil moiety is metabolized to acetaldehyde and acetic acid.
Excretion
Cefuroxime is excreted unchanged in the urine; in adults, approximately 50% of the administered dose is recovered in the urine within 12 hours. The pharmacokinetics of cefuroxime in pediatric subjects have not been studied. Until further data are available, the renal elimination of cefuroxime axetil established in adults should not be extrapolated to pediatric subjects.
Specific Populations
Patients with Renal Impairment: In a trial of 28 adults with normal renal function or severe renal impairment (creatinine clearance <30 mL/min), the elimination half-life was prolonged in relation to severity of renal impairment. Prolongation of the dosage interval is recommended in adult patients with creatinine clearance <30 mL/min [see DOSAGE AND ADMINISTRATION (2.5)].
Geriatric Patients: In a trial of 20 elderly subjects (mean age = 83.9 years) having a mean creatinine clearance of 34.9 mL/min, the mean serum elimination half-life was prolonged to 3.5 hours; however, despite the lower elimination of cefuroxime in geriatric patients, dosage adjustment based on age is not necessary [see USE IN SPECIFIC POPULATIONS (8.5)].
Drug Interaction Studies
Concomitant administration of probenecid with cefuroxime axetil tablets increases the cefuroxime area under the serum concentration versus time curve and maximum serum concentration by 50% and 21%, respectively [see DRUG INTERACTIONS (7.2)].
12.4 Microbiology
Cefuroxime axetil is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefuroxime axetil has activity in the presence of some β-lactamases, both penicillinases and cephalosporinases, of gram-negative and gram-positive bacteria.
Resistance
Resistance to cefuroxime axetil is primarily through hydrolysis by β-lactamase, alteration of penicillin-binding proteins (PBPs), decreased permeability, and the presence of bacterial efflux pumps.
Susceptibility to cefuroxime axetil will vary with geography and time; local susceptibility data should be consulted, if available. Beta-lactamase-negative, ampicillin-resistant (BLNAR) isolates of H. influenzae should be considered resistant to cefuroxime axetil.
Antimicrobial Activity
Cefuroxime axetil has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections [see INDICATIONS AND USAGE (1)]:
Aerobic Bacteria:
Gram-positive bacteria
● Staphylococcus aureus (methicillin-susceptible isolates only)
● Streptococcus pneumoniae
● Streptococcus pyogenes
Gram-negative bacteria
● Escherichia colia
● Klebsiella pneumoniaea
● Haemophilus influenzae
● Haemophilus parainfluenzae
● Moraxella catarrhalis
● Neisseria gonorrhoeae
a Most extended spectrum β-lactamase (ESBL)-producing and carbapenemase-producing isolates are resistant to cefuroxime axetil.
Spirochetes
● Borrelia burgdorferi
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for cefuroxime against isolates of similar genus or organism group.
However, the efficacy of cefuroxime axetil in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Aerobic Bacteria:
Gram-positive bacteria
● Staphylococcus epidermidis (methicillin-susceptible isolates only)
● Staphylococcus saprophyticus (methicillin-susceptible isolates only)
● Streptococcus agalactiae
Gram-negative bacteria
● Morganella morganii
● Proteus inconstans
● Proteus mirabilis
● Providencia rettgeri
Anaerobic bacteria:
Gram-positive bacteria
● Peptococcus niger
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Although lifetime studies in animals have not been performed to evaluate carcinogenic potential, no mutagenic activity was found for cefuroxime axetil in a battery of bacterial mutation tests. Positive results were obtained in an in vitro chromosome aberration assay; however, negative results were found in an in vivo micronucleus test at doses up to 1.5 g/kg. Fertility studies in rats (males dosed for 70 days prior to and through mating; females dosed 21 days prior to mating through lactation) at doses up to 1,000 mg/kg/day (9 times the MRHD based on body surface area) have revealed no adverse effects on fertility.
-
14 CLINICAL STUDIES
14.1 Acute Bacterial Maxillary Sinusitis
One adequate and well-controlled trial was performed in subjects with acute bacterial maxillary sinusitis. In this trial, each subject had a maxillary sinus aspirate collected by sinus puncture before treatment was initiated for presumptive acute bacterial sinusitis. All subjects had radiographic and clinical evidence of acute maxillary sinusitis. In the trial, the clinical effectiveness of cefuroxime axetil in treating acute maxillary sinusitis was comparable to an oral antimicrobial agent containing a specific β-lactamase inhibitor. However, microbiology data demonstrated cefuroxime axetil to be effective in treating acute bacterial maxillary sinusitis due only to Streptococcus pneumoniae or non-β-lactamase–producing Haemophilus influenzae. Insufficient numbers of β-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis isolates were obtained in this trial to adequately evaluate the effectiveness of cefuroxime axetil in treating acute bacterial maxillary sinusitis due to these 2 organisms.
This trial randomized 317 adult subjects, 132 subjects in the U. S. and 185 subjects in South America. Table 11 shows the results of the intent-to-treat analysis.
Table 11. Clinical Effectiveness of Cefuroxime Axetil Tablets in the Treatment of Acute Bacterial Maxillary Sinusitis - * 95% confidence interval around the success difference [-0.08, +0.32].
- † 95% confidence interval around the success difference [-0.10, +0.16].
U.S. Subjects*
South American Subjects†
Cefuroxime Axetil Tablets 250 mg Twice Daily
(n = 49)
Controlc
(n = 43)
Cefuroxime Axetil Tablets 250 mg Twice Daily
(n = 49)
Controlc
(n = 43)
Clinical success
(cure + improvement)
65%
53%
77%
74%
Clinical cure
53%
44%
72%
64%
Clinical improvement
12%
9%
5%
10%
In this trial and in a supporting maxillary puncture trial, 15 evaluable subjects had non β-lactamase–producing Haemophilus influenzae as the identified pathogen. Of these, 67% (10/15) had this pathogen eradicated. Eighteen (18) evaluable subjects had Streptococcus pneumoniae as the identified pathogen. Of these, 83% (15/18) had this pathogen eradicated.
14.2 Early Lyme Disease
Two adequate and well-controlled trials were performed in subjects with early Lyme disease. All subjects presented with physician-documented erythema migrans, with or without systemic manifestations of infection. Subjects were assessed at 1 month posttreatment for success in treating early Lyme disease (Part I) and at 1 year posttreatment for success in preventing the progression to the sequelae of late Lyme disease (Part II).
A total of 355 adult subjects (181 treated with cefuroxime axetil and 174 treated with doxycycline) were randomized in the 2 trials, with diagnosis of early Lyme disease confirmed in 79% (281/355). The clinical diagnosis of early Lyme disease in these subjects was validated by 1) blinded expert reading of photographs, when available, of the pretreatment erythema migrans skin lesion, and 2) serologic confirmation (using enzyme-linked immunosorbent assay [ELISA] and immunoblot assay ["Western" blot]) of the presence of antibodies specific to Borrelia burgdorferi, the etiologic agent of Lyme disease. The efficacy data in Table 12 are specific to this "validated" patient subset, while the safety data below reflect the entire patient population for the 2 trials. Clinical data for evaluable subjects in the "validated" patient subset are shown in Table 12.
Table 12. Clinical Effectiveness of Cefuroxime Axetil Tablets Compared with Doxycycline in the Treatment of Early Lyme Disease - * 95% confidence interval around the satisfactory difference for Part I (-0.08, +0.05).
- † 95% confidence interval around the satisfactory difference for Part II (-0.13, +0.07).
- ‡ n's include subjects assessed as unsatisfactory clinical outcomes (failure + recurrence) in Part I (Cefuroxime Axetil Tablets - 11 [5 failure, 6 recurrence]; doxycycline - 8 [6 failure, 2 recurrence]).
- § Satisfactory clinical outcome includes cure + improvement (Part I) and success + improvement (Part II).
Part I
(1 Month after 20 Days of Treatment)*
Part II
(1 Year after 20 Days of Treatment)†
Cefuroxime Axetil Tablets 500 mg Twice Daily
(n = 125)
Doxycycline 100 mg 3 Times Daily
(n = 108)
Cefuroxime Axetil Tablets 500 mg Twice Daily
(n = 105‡)
Doxycycline 100 mg 3 Times Daily (n = 83‡)
Satisfactory clinical outcome§
91%
93%
84%
87%
Clinical cure/success
72%
73%
73%
73%
Clinical improvement
19%
19%
10%
13%
Cefuroxime axetil and doxycycline were effective in prevention of the development of sequelae of late Lyme disease.
While the incidence of drug-related gastrointestinal adverse reactions was similar in the 2 treatment groups (cefuroxime axetil - 13%; doxycycline - 11%), the incidence of drug-related diarrhea was higher in the cefuroxime axetil arm versus the doxycycline arm (11% versus 3%, respectively).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Cefuroxime axetil tablets USP, 250 mg of cefuroxime (as cefuroxime axetil), are white to off-white, capsule-shaped, film-coated tablets with "LUPIN" debossed on one side and "302" on the other side, supplied in bottles of 20 and 60.
20s Bottle NDC: 68180-302-20
60s Bottle NDC: 68180-302-60
Cefuroxime axetil tablets USP, 500 mg of cefuroxime (as cefuroxime axetil), are white to off-white, capsule-shaped, film-coated tablets with "LUPIN" debossed on one side and "303" on the other side, supplied in bottles of 20 and 60.
20s Bottle NDC: 68180-303-20
60s Bottle NDC: 68180-303-60
Store the tablets at 20 to 25°C (68 to 77°F) [See USP Controlled Room Temperature]. Replace cap securely after each opening.
-
17 PATIENT COUNSELING INFORMATION
Inform patients that cefuroxime axetil is a cephalosporin that can cause allergic reactions in some individuals [see WARNINGS AND PRECAUTIONS (5.1)].
Clostridium difficile-Associated Diarrhea
Inform patients that diarrhea is a common problem caused by antibacterials, and it usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as 2 or more months after having taken their last dose of the antibacterial. If this occurs, advise patients to contact their physician as soon as possible.
Crushing Tablets
Instruct patients to swallow the tablet whole, without crushing the tablet. Patients who cannot swallow the tablet whole should receive the oral suspension.
Drug Resistance
Inform patients that antibacterial drugs, including cefuroxime axetil, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefuroxime axetil is prescribed to treat a bacterial infection, inform patients that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefuroxime axetil or other antibacterial drugs in the future.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States.
Manufactured by:
Lupin Limited
Mandideep 462 046
INDIA.
Revised: July 2019 ID#:260856
-
PRINCIPAL DISPLAY PANEL
Cefuroxime Axetil Tablets USP, 250 mg
Bottle of 20s
NDC: 68180-302-20

Cefuroxime Axetil Tablets USP, 500 mg
Bottle of 20s
NDC: 68180-303-20
-
INGREDIENTS AND APPEARANCE
CEFUROXIME AXETIL
cefuroxime axetil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68180-302 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFUROXIME AXETIL (UNII: Z49QDT0J8Z) (CEFUROXIME - UNII:O1R9FJ93ED) CEFUROXIME 250 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (white to off white) Score no score Shape OVAL (capsule shaped) Size 15mm Flavor Imprint Code LUPIN;302 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68180-302-20 20 in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2008 2 NDC: 68180-302-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065135 10/08/2008 CEFUROXIME AXETIL
cefuroxime axetil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68180-303 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFUROXIME AXETIL (UNII: Z49QDT0J8Z) (CEFUROXIME - UNII:O1R9FJ93ED) CEFUROXIME 500 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (white to off white) Score no score Shape OVAL (capsule shaped) Size 19mm Flavor Imprint Code LUPIN;303 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68180-303-20 20 in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2008 2 NDC: 68180-303-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/08/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065135 10/08/2008 Labeler - Lupin Pharmaceuticals, Inc. (089153071) Registrant - LUPIN LIMITED (675923163) Establishment Name Address ID/FEI Business Operations LUPIN LIMITED 725504448 MANUFACTURE(68180-302, 68180-303) , PACK(68180-302, 68180-303)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.