MARAVIROC tablet, film coated
Maraviroc by
Drug Labeling and Warnings
Maraviroc by is a Prescription medication manufactured, distributed, or labeled by i3 Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MARAVIROC TABLETS safely and effectively. See full prescribing information for MARAVIROC TABLETS.
MARAVIROC tablets, for oral use
Initial U.S. Approval: 2007WARNING: HEPATOTOXICITY
See full prescribing information for complete boxed warning.
Hepatotoxicity has been reported which may be preceded by severe rash or other features of a systemic allergic reaction (e.g., fever, eosinophilia, or elevated IgE). ( 5.1)
Immediately evaluate patients with signs or symptoms of hepatitis or allergic reaction. ( 5.1)
INDICATIONS AND USAGE
Maraviroc is a CCR5 co-receptor antagonist indicated in combination with other antiretroviral agents for the treatment of only CCR5-tropic HIV-1 infection in adults and pediatric patients 2 years of age and older weighing at least 10 kg. ( 1)
Limitations of Use:
Not recommended in patients with dual/mixed- or CXCR4-tropic HIV-1. ( 1)
DOSAGE AND ADMINISTRATION
Prior to initiation of Maraviroc for treatment of HIV-1 infection, test all patients for CCR5 tropism using a highly sensitive tropism assay. ( 2.1)
Maraviroc tablets are taken twice daily by mouth and may be taken with or without food. Maraviroc must be given in combination with other antiretroviral medications. ( 2.2)
Recommended Dosage in Adult Patients: ( 2.3)
Concomitant Medications Dosage of Maraviroc When given with potent cytochrome P450 (CYP)3A inhibitors (with or without potent CYP3A inducers) including PIs (except tipranavir/ritonavir) ( 2.3, 7.1) 150 mg twice daily With NRTIs, tipranavir/ritonavir, nevirapine, raltegravir, and other drugs that are not potent CYP3A inhibitors or CYP3A inducers ( 2.3, 7.1) 300 mg twice daily With potent and moderate CYP3A inducers including efavirenz (without a potent CYP3A inhibitor) ( 2.3, 7.1) 600 mg twice daily A more complete list of coadministered drugs is listed in Dosage and Administration. ( 2)
Recommended Dosage in Pediatric Patients 2 years and older and weighing at Least 10 kg: Administer twice daily. Dosage should be based on body weight (kg) and concomitant medications and should not exceed the recommended adult dose. ( 2.4)
Recommended Dosage in Patients with Renal Impairment: Dose adjustment may be necessary in adult patients with renal impairment. ( 2.5)
DOSAGE FORMS AND STRENGTHS
Tablets: 150 mg and 300 mg. ( 3)
CONTRAINDICATIONS
Maraviroc is contraindicated in patients with severe renal impairment or end-stage renal disease (ESRD) (creatinine clearance [CrCl] less than 30 mL per minute) who are concomitantly taking potent CYP3A inhibitors or inducers. ( 4)
WARNINGS AND PRECAUTIONS
Hepatotoxicity accompanied by severe rash or systemic allergic reaction, including potentially life- threatening events, has been reported. Hepatic laboratory parameters including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubinshould be obtained prior to starting Maraviroc and at other time points during treatment as clinically indicated. If rash or symptoms or signs of hepatitis or allergic reaction develop, hepatic laboratory parameters should be monitored and discontinuation of treatment should be considered. When administering Maraviroc to patients with pre-existing liver dysfunction or who are co-infected with hepatitis B and/or C virus, additional monitoring may be warranted. ( 5.1)
Severe and potentially life-threatening skin and hypersensitivity reactions have been reported in patients taking Maraviroc. This includes cases of Stevens-Johnson syndrome, hypersensitivity reaction, and toxic epidermal necrolysis. Immediately discontinue Maraviroc and other suspected agents if signs or symptoms of severe skin or hypersensitivity reactions develop and monitor clinical status, including liver aminotransferases, closely. ( 5.2)
More cardiovascular events, including myocardial ischemia and/or infarction, were observed in treatment-experienced subjects who received Maraviroc. Additional monitoring may be warranted. ( 5.3)
If patients with severe renal impairment or ESRD receiving Maraviroc (without concomitant CYP3A inducers or inhibitors) experience postural hypotension, the dose of Maraviroc should be reduced from 300 mg twice daily to 150 mg twice daily. ( 5.3)
ADVERSE REACTIONS
The most common adverse events in treatment-experienced adult subjects (greater than 8% incidence) which occurred at a higher frequency compared with placebo are upper respiratory tract infections, cough, pyrexia, rash, and dizziness. ( 6.1)
The most common adverse events in treatment-naive adult subjects (greater than 8% incidence) which occurred at a higher frequency than the comparator arm are upper respiratory tract infections, bronchitis, flatulence, bloating and distention, upper respiratory tract signs and symptoms, and gastrointestinal atonic and hypomotility disorders. ( 6.1)
The most common adverse reactions in treatment-experienced pediatric subjects (greater than or equal to 3% incidence) are vomiting, abdominal pain, diarrhea, nausea, and dizziness. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact i3 Pharmaceuticals, LLC at 1-844-874-7353 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
Coadministration with CYP3A inhibitors, including protease inhibitors (except tipranavir/ritonavir), will increase the concentration of Maraviroc. ( 7.1)
Coadministration with CYP3A inducers, including efavirenz, may decrease the concentration of Maraviroc. ( 7.1)
Coadministration with St. John’s wort is not recommended. ( 7.1)
USE IN SPECIFIC POPULATIONS
Lactation: Women infected with HIV should be instructed not to breastfeed due to the potential for HIV transmission. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HEPATOTOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Testing prior to Initiation of Maraviroc
2.2 General Dosing Recommendations
2.3 Recommended Dosage in Adult Patients with Normal Renal Function
2.4 Recommended Dosage in Pediatric Patients with Normal Renal Function
2.5 Recommended Dosage in Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
5.2 Severe Skin and Hypersensitivity Reactions
5.3 Cardiovascular Events
5.4 Immune Reconstitution Syndrome
5.5 Potential Risk of Infection
5.6 Potential Risk of Malignancy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Concomitant Drugs on the Pharmacokinetics of Maraviroc
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Studies in Adult Subjects
14.2 Clinical Studies in Pediatric Subjects
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: HEPATOTOXICITY
Hepatotoxicity has been reported with use of Maraviroc. Severe rash or evidence of a systemic allergic reaction (e.g., fever, eosinophilia, or elevated IgE) prior to the development of hepatotoxicity may occur. Patients with signs or symptoms of hepatitis or allergic reaction following use of Maraviroc should be evaluated immediately [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
Maraviroc is indicated in combination with other antiretroviral agents for the treatment of only CCR5‑tropic human immunodeficiency virus type 1 (HIV‑1) infection in adult and pediatric patients 2 years of age and older weighing at least 10 kg.
Limitations of Use
- Maraviroc is not recommended in patients with dual/mixed- or CXCR4-tropic HIV- 1 [see Microbiology (12.4)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 Testing prior to Initiation of Maraviroc
Prior to initiation of Maraviroc for treatment of HIV-1 infection, test all patients for CCR5 tropism using a highly sensitive tropism assay. Maraviroc is recommended for patients with only CCR5-tropic HIV-1 infection. Outgrowth of pre-existing low-level CXCR4- or dual/mixed-tropic HIV-1 not detected by tropism testing at screening has been associated with virologic failure on Maraviroc [see Microbiology (12.4), Clinical Studies (14.1)].
Monitor patients for alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin prior to initiation of Maraviroc and at other time points during treatment as clinically indicated [see Warnings and Precautions (5.1)].
2.2 General Dosing Recommendations
- Maraviroc tablets are taken twice daily by mouth and may be taken with or without food.
- Maraviroc must be given in combination with other antiretroviral medications.
- The recommended dosage of Maraviroc differs based on concomitant medications due to drug interactions.
2.3 Recommended Dosage in Adult Patients with Normal Renal Function
Table 1 displays oral dosage of Maraviroc based on different concomitant medications [see Drug Interactions (7.1)].
Table 1. Recommended Dosage in Adults - * Potent CYP3A inhibitors (with or without a potent CYP3A inducer) including: clarithromycin, cobicistat, elvitegravir/ritonavir, itraconazole, ketoconazole, nefazodone, protease inhibitors (except tipranavir/ritonavir), telithromycin.
- † Noninteracting concomitant medications include all medications that are not potent CYP3A inhibitors or inducers such as: dolutegravir, enfuvirtide, nevirapine, all nucleoside reverse transcriptase inhibitors (NRTIs), raltegravir, and tipranavir/ritonavir.
- ‡ Potent and moderate CYP3A inducers (without a potent CYP3A inhibitor) including: carbamazepine, efavirenz, etravirine, phenobarbital, phenytoin, and rifampin.
Concomitant Medications Dosage of Maraviroc Potent cytochrome P450 (CYP)3A inhibitors (with or without a potent CYP3A inducer) * 150 mg twice daily Noninteracting concomitant medications † 300 mg twice daily Potent and moderate CYP3A inducers (without a potent CYP3A inhibitor) ‡ 600 mg twice daily 2.4 Recommended Dosage in Pediatric Patients with Normal Renal Function
The recommended dosage of Maraviroc should be based on body weight (kg) and should not exceed the recommended adult dose. The recommended dosage also differs based on concomitant medications due to drug interactions (Table 2 and Table 3) [see Drug Interactions (7.1), Use in Specific Populations (8.4)].
Before prescribing Maraviroc tablets, assess children for the ability to swallow tablets. If a child is unable to reliably swallow Maraviroc tablets, the oral solution formulation should be prescribed.
The recommended oral dosage of Maraviroc tablets in pediatric patients aged 2 years and older weighing at least 10 kg is presented in Table 2.
Table 2. Recommended Dosage in Pediatric Patients Aged 2 Years and Older Weighing at Least 10 kg (Tablets) - * Potent CYP3A inhibitors (with or without a CYP3A inducer) including: clarithromycin, cobicistat, elvitegravir/ritonavir, itraconazole, ketoconazole, nefazodone, protease inhibitors (except tipranavir/ritonavir), telithromycin.
- † Noninteracting concomitant medications including all medications that are not potent CYP3A inhibitors or inducers such as: dolutegravir, enfuvirtide, nevirapine, all NRTIs, raltegravir, and tipranavir/ritonavir.
- ‡ Potent and moderate CYP3A inducers (without a potent CYP3A inhibitor) including: carbamazepine, efavirenz, etravirine, phenobarbital, phenytoin, and rifampin.
- § Insufficient data are available to recommend use.
Concomitant Medications Dosage of Maraviroc Based on Weight 10 kg to
<14 kg14 kg to
<20 kg20 kg to
<30 kg30 kg to
<40 kg≥40 kg Potent CYP3A inhibitors (with or without a CYP3A inducer) * 50 mg twice daily 50 mg twice daily 75 mg twice daily 100 mg twice daily 150 mg twice daily Noninteracting concomitant medications † 150 mg twice daily 200 mg twice daily 200 mg twice daily 300 mg twice daily 300 mg twice daily Potent and moderate CYP3A inducers (without a potent CYP3A inhibitor) ‡ Not recommended § The recommended oral dosage of maraviroc oral solution in pediatric patients weighing at least 10 kg is presented in Table 3.
Table 3. Recommended Dosage in Pediatric Patients Weighing at Least 10 kg (Oral Solution) - * Potent CYP3A inhibitors (with or without a CYP3A inducer) including: clarithromycin, cobicistat, elvitegravir/ritonavir, itraconazole, ketoconazole, nefazodone, protease inhibitors (except tipranavir/ritonavir), telithromycin.
- † Noninteracting concomitant medications including all medications that are not potent CYP3A inhibitors or inducers such as: dolutegravir, enfuvirtide, nevirapine, all NRTIs, raltegravir, and tipranavir/ritonavir.
- ‡ Potent and moderate CYP3A inducers (without a potent CYP3A inhibitor) including: carbamazepine, efavirenz, etravirine, phenobarbital, phenytoin, and rifampin.
- § Insufficient data are available to recommend use.
Concomitant Medications
Dosage (Volume of Solution) of Maraviroc Based on Weight
10 kg to
<14 kg
14 kg to
<20 kg
20 kg to
<30 kg
30 kg to
<40 kg
≥40 kg
Potent CYP3A inhibitors (with or without a CYP3A inducer) *
50 mg (2.5 mL) twice daily
50 mg (2.5 mL) twice daily
80 mg (4 mL) twice daily
100 mg (5 mL) twice daily
150 mg (7.5 mL) twice daily
Noninteracting concomitant medications †
150 mg (7.5 mL) twice daily
200 mg (10 mL) twice daily
200 mg (10 mL) twice daily
300 mg (15 mL) twice daily
300 mg (15 mL) twice daily
Potent and moderate CYP3A inducers (without a potent CYP3A inhibitor) ‡
Not recommended §
Administer the oral solution using the appropriate oral dosing syringe: for doses greater than 2.5 mL, use the 10-mL syringe.
2.5 Recommended Dosage in Patients with Renal Impairment
Adult Patients
Table 4 provides dosing recommendations for patients based on renal function and concomitant medications.
Table 4. Recommended Dosage in Adults Based on Renal Function - * Potent CYP3A inhibitors (with or without a CYP3A inducer) including: clarithromycin, cobicistat, elvitegravir/ritonavir, itraconazole, ketoconazole, nefazodone, protease inhibitors (except tipranavir/ritonavir), telithromycin.
- † Noninteracting concomitant medications include all medications that are not potent CYP3A inhibitors or inducers such as: dolutegravir, enfuvirtide, nevirapine, all NRTIs, raltegravir, and tipranavir/ritonavir.
- ‡ Dosage of Maraviroc should be reduced to 150 mg twice daily if there are any symptoms of postural hypotension [see Contraindications (4), Warnings and Precautions (5.3)].
- § Potent and moderate CYP3A inducers (without a potent CYP3A inhibitor) including: carbamazepine, efavirenz, etravirine, phenobarbital, phenytoin, and rifampin.
Concomitant Medications Dosage of Maraviroc Based on Renal Function Normal (CrCl >80
mL/min)Mild (CrCl >50
and ≤80
mL/min)Moderate (CrCl ≥30 and ≤50 mL/min)
Severe
(CrCl <30
mL/min)End-Stage
Renal Disease on
Regular Hemodialysis
Potent CYP3A inhibitors (with or without a CYP3A inducer) * 150 mg
twice daily
150 mg
twice daily
150 mg
twice daily
Contraindicated
Contraindicated
Noninteracting concomitant medications † 300 mg
twice daily
300 mg
twice daily
300 mg
twice daily
300 mg
twice daily
300 mg
twice daily ‡
Potent and moderate CYP3A inducers (without a potent CYP3A inhibitor) § 600 mg
twice daily
600 mg
twice daily
600 mg
twice daily
Contraindicated Contraindicated
CrCl = Creatinine Clearance.
Pediatric Patients
There are no data to recommend specific doses of Maraviroc in pediatric patients with mild or moderate renal impairment [see Use in Specific Populations (8.6)] . Additionally, Maraviroc is contraindicated for pediatric patients with severe renal impairment or end- stage renal disease (ESRD) on regular hemodialysis who are receiving potent CYP3A inhibitors or inducers [see Contraindications (4)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Maraviroc is contraindicated in patients with severe renal impairment or ESRD (creatinine clearance [CrCl] less than 30 mL per minute) who are concomitantly taking potent CYP3A inhibitors or inducers [see Warnings and Precautions (5.3)]. -
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
Hepatotoxicity with allergic features including life-threatening events has been reported in clinical trials and postmarketing. Severe rash or evidence of systemic allergic reaction including drug-related rash with fever, eosinophilia, elevated IgE, or other systemic symptoms have been reported in conjunction with hepatotoxicity [see Warnings and Precautions (5.2)] . These events occurred approximately 1 month after starting treatment. Among reported cases of hepatitis, some were observed in the absence of allergic features or with no pre-existing hepatic disease.
Appropriate laboratory testing including ALT, AST, and bilirubin should be conducted prior to initiating therapy with Maraviroc and at other time points during treatment as clinically indicated. Hepatic laboratory parameters should be obtained in any patient who develops rash, or signs or symptoms of hepatitis, or allergic reaction. Discontinuation of Maraviroc should be considered in any patient with signs or symptoms of hepatitis, or with increased liver transaminases combined with rash or other systemic symptoms.
When administering Maraviroc to patients with pre-existing liver dysfunction or who are co-infected with hepatitis B and/or C virus, additional monitoring may be warranted. The safety and efficacy of Maraviroc have not been specifically studied in patients with significant underlying liver disorders.
5.2 Severe Skin and Hypersensitivity Reactions
Severe, potentially life-threatening skin and hypersensitivity reactions have been reported in patients taking Maraviroc, in most cases concomitantly with other drugs associated with these reactions. These include cases of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) [see Adverse Reactions (6.2)] . The cases were characterized by features including rash, constitutional findings, and sometimes organ dysfunction, including hepatic failure. Discontinue Maraviroc and other suspected agents immediately if signs or symptoms of severe skin or hypersensitivity reactions develop (including, but not limited to, severe rash or rash accompanied by fever, malaise, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema, lip swelling, eosinophilia). Delay in stopping treatment with Maraviroc or other suspect drugs after the onset of rash may result in a life-threatening reaction. Clinical status, including liver aminotransferases, should be monitored and appropriate therapy initiated.
5.3 Cardiovascular Events
Eleven subjects (1.3%) who received Maraviroc had cardiovascular events, including myocardial ischemia and/or infarction, during the Phase 3 trials in treatment‑experienced subjects (total exposure 609 patient‑years [300 on Maraviroc once daily + 309 on Maraviroc twice daily]), while no subjects who received placebo had such events (total exposure 111 patient‑years). These subjects generally had cardiac disease or cardiac risk factors prior to use of Maraviroc, and the relative contribution of Maraviroc to these events is not known.
In the Phase 2b/3 trial in treatment‑naive adult subjects, 3 subjects (0.8%) who received Maraviroc had events related to ischemic heart disease and 5 subjects (1.4%) who received efavirenz had such events (total exposure 506 and 508 patient‑years for Maraviroc and efavirenz, respectively).
When Maraviroc was administered to healthy volunteers at doses higher than the recommended dose, symptomatic postural hypotension was seen at a greater frequency than in placebo. However, when Maraviroc was given at the recommended dose in HIV-1–infected adult subjects in Phase 3 trials, postural hypotension was seen at a rate similar to placebo (approximately 0.5%).
Patients with cardiovascular comorbidities, risk factors for postural hypotension, or receiving concomitant medication known to lower blood pressure, could be at increased risk of cardiovascular adverse events triggered by postural hypotension. Additional monitoring may be warranted.
Postural Hypotension in Patients with Renal Impairment
An increased risk of postural hypotension may occur in patients with severe renal insufficiency or in those with ESRD due to increased maraviroc exposure in some patients. Maraviroc should be used in patients with severe renal impairment or ESRD only if they are not receiving a concomitant potent CYP3A inhibitor or inducer. However, the use of Maraviroc in these patients should only be considered when no alternative treatment options are available. If adult patients with severe renal impairment or ESRD experience any symptoms of postural hypotension while taking 300 mg twice daily, the dose should be reduced to 150 mg twice daily [see Dosage and Administration (2.5)] .
5.4 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including Maraviroc. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as infection with Mycobacterium aviuminfection, cytomegalovirus, Pneumocystis jirovecii pneumonia[PCP], tuberculosis, or reactivation of Herpes simplexand Herpes zoster), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.5 Potential Risk of Infection
Maraviroc antagonizes the CCR5 co-receptor located on some immune cells, and therefore could potentially increase the risk of developing infections. The overall incidence and severity of infection, as well as AIDS-defining category C infections, were comparable in the treatment groups during the Phase 3 adult treatment‑experienced trials of Maraviroc. While there was a higher rate of certain upper respiratory tract infections reported in the treatment arm receiving Maraviroc compared with placebo (23% versus 13%), there was a lower rate of pneumonia (2% versus 5%) reported in subjects receiving Maraviroc. A higher incidence of Herpes virus infections (11 per 100 patient‑years) was also reported in the treatment arm receiving Maraviroc when adjusted for exposure compared with placebo (8 per 100 patient‑years).
In the Phase 2b/3 trial in treatment‑naive adult subjects, the incidence of AIDS-defining Category C events when adjusted for exposure was 1.8 for Maraviroc compared with 2.4 for efavirenz per 100 patient‑years of exposure.
Patients should be monitored closely for evidence of infections while receiving Maraviroc
5.6 Potential Risk of Malignancy
While no increase in malignancy has been observed with Maraviroc, due to this drug’s mechanism of action, it could affect immune surveillance and lead to an increased risk of malignancy.
The exposure-adjusted rate for malignancies per 100 patient‑years of exposure in adult treatment‑experienced trials was 4.6 for Maraviroc compared with 9.3 on placebo. In treatment‑naive adult subjects, the rates were 1.0 and 2.4 per 100 patient‑years of exposure for Maraviroc and efavirenz, respectively.
Long-term follow-up is needed to more fully assess this risk.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Hepatotoxicity [see Boxed Warning, Warnings and Precautions (5.1)]
- Severe Skin and Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Cardiovascular Events [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Experience in Adult Subjects
Treatment‑Experienced Subjects:The safety profile of Maraviroc is primarily based on 840 HIV-1infected subjects who received at least 1 dose of Maraviroc during two Phase 3 trials. A total of 426 of these subjects received the indicated twice‑daily dosing regimen.
Assessment of treatment‑emergent adverse events is based on the pooled data from 2 trials in subjects with CCR5-tropic HIV‑1 (A4001027 and A4001028). The median duration of therapy with Maraviroc for subjects in these trials was 48 weeks, with the total exposure on Maraviroc twice daily at 309 patient‑years versus 111 patient‑years on placebo each administered with optimized background therapy (OBT). The population was 89% male and 84% white, with mean age of 46 years (range: 17 to 75 years). Subjects received dose equivalents of 300 mg maraviroc once or twice daily.
The most common adverse events reported with twice‑daily therapy with Maraviroc with frequency rates higher than placebo, regardless of causality, were upper respiratory tract infections, cough, pyrexia, rash, and dizziness. In these 2 trials, the rate of discontinuation due to adverse events was 5% for subjects who received Maraviroc twice daily + OBT as well as those who received placebo + OBT. Most of the adverse events reported were judged to be mild to moderate in severity. The data described below occurred with twice‑daily dosing of Maraviroc.
The total numbers of subjects reporting infections were 233 (55%) and 84 (40%) in the group receiving Maraviroc twice daily and the placebo group, respectively. Correcting for the longer duration of exposure on Maraviroc compared with placebo, the exposure‑adjusted frequency (rate per 100 subject‑years) of these events was 133 for both Maraviroc twice daily and placebo.
Dizziness or postural dizziness occurred in 8% of subjects on either Maraviroc or placebo, with 2 subjects (0.5%) on Maraviroc permanently discontinuing therapy (1 due to syncope, 1 due to orthostatic hypotension) versus 1 subject on placebo (0.5%) permanently discontinuing therapy due to dizziness.
Treatment-emergent adverse events, regardless of causality, from Trials A4001027 and A4001028 are summarized in Table 5. Selected events occurring at greater than or equal to 2% of subjects and at a numerically higher rate in subjects treated with Maraviroc are included; events that occurred at the same or higher rate on placebo are not displayed.
Table 5. Selected Treatment-Emergent Adverse Events (All Causality) ≥2% on Maraviroc (and at a Higher Rate Compared with Placebo) in Trials A4001027 and A4001028 (Pooled Analysis, 48 Weeks) - * 300-mg dose equivalent.
- † PYE = Patient-years of exposure.
Body System/
Adverse Event
Maraviroc
Twice Daily *Placebo (n = 426)
%Exposure- Adjusted Rate (per 100 pt- yrs)
PYE = 309 †(n = 209)
%Exposure- Adjusted Rate (per 100 pt- yrs)
PYE = 111 †Eye Disorders Conjunctivitis 2 3 1 3 Ocular infections, inflammations, and associated manifestations 2 3 1 2 Gastrointestinal Disorders Constipation 6 9 3 6 General Disorders and Administration Site Conditions Pyrexia 13 20 9 17 Pain and discomfort 4 5 3 5 Infections and Infestations Upper respiratory tract infection 23 37 13 27 Herpes infection 8 11 4 8 Sinusitis 7 10 3 6 Bronchitis 7 9 5 9 Folliculitis 4 5 2 4 Anogenital warts 2 3 1 3 Influenza 2 3 0.5 1 Otitis media 2 3 0.5 1 Metabolism and Nutrition Disorders Appetite disorders 8 11 7 13 Musculoskeletal and Connective Tissue Disorders Joint-related signs and symptoms 7 10 3 5 Muscle pains 3 4 0.5 1 Neoplasms Benign, Malignant, and Unspecified Skin neoplasms benign 3 4 1 3 Nervous System Disorders Dizziness/postural dizziness 9 13 8 17 Paresthesias and dysesthesias 5 7 3 6 Sensory abnormalities 4 6 1 3 Disturbances in consciousness 4 5 3 6 Peripheral neuropathies 4 5 3 6 Psychiatric Disorders Disturbances in initiating and maintaining sleep 8 11 5 10 Depressive disorders 4 6 3 5 Anxiety symptoms 4 5 3 7 Renal and Urinary Disorders Bladder and urethral symptoms 5 7 1 3 Urinary tract signs and symptoms 3 4 1 3 Respiratory, Thoracic, and Mediastinal Disorders Coughing and associated symptoms 14 21 5 10 Upper respiratory tract signs and symptoms 6 9 3 6 Nasal congestion and inflammations 4 6 3 5 Breathing abnormalities 4 5 2 5 Paranasal sinus disorders 3 4 0.5 1 Skin and Subcutaneous Tissue Disorders Rash 11 16 5 11 Apocrine and eccrine gland disorders 5 7 4 7.5 Pruritus 4 5 2 4 Lipodystrophies 3 5 0.5 1 Erythema 2 3 1 2 Vascular Disorders Vascular hypertensive disorders 3 4 2 4 Laboratory Abnormalities:Table 6 shows the treatment-emergent Grade 3-4 laboratory abnormalities that occurred in greater than 2% of subjects receiving Maraviroc.
Table 6. Maximum Shift in Laboratory Test Values (without Regard to Baseline) ≥2% of Grade 3-4 Abnormalities (ACTG Criteria) in Trials A4001027 and A4001028 (Pooled Analysis, 48 Weeks) - * Percentages based on total subjects evaluated for each laboratory parameter.
Laboratory Parameter Preferred Term Limit Maraviroc Twice Daily + OBT
(n = 421) *%
Placebo + OBT
(n = 207) *
%Aspartate aminotransferase >5.0 x ULN 4.8 2.9 Alanine aminotransferase >5.0 x ULN 2.6 3.4 Total bilirubin >2.5 x ULN 5.5 5.3 Amylase >2.0 x ULN 5.7 5.8 Lipase >2.0 x ULN 4.9 6.3 Absolute neutrophil count <750/mm 3 4.3 2.4 ULN = Upper limit of normal; OBT = optimized background therapy.
Treatment‑Naive Subjects: Treatment-Emergent Adverse Events:Treatment-emergent adverse events, regardless of causality, from Trial A4001026, a double-blind, comparative, controlled trial in which 721 treatment-naive subjects received Maraviroc 300 mg twice daily (n = 360) or efavirenz 600 mg once daily (n = 361) in combination with lamivudine/zidovudine (COMBIVIR) for 96 weeks, are summarized in Table 7. Selected events occurring in greater than or equal to 2% of subjects and at a numerically higher rate in subjects treated with Maraviroc are included; events that occurred at the same or higher rate on efavirenz are not displayed.
Table 7. Selected Treatment-Emergent Adverse Events (All Causality) ≥2% on Maraviroc (and at a Higher Rate Compared with Efavirenz) in Trial A4001026 (96 Weeks) Body System/
Adverse Event
Maraviroc
300 mg Once Daily +
Lamivudine/Zidovudine
(n = 360)
%Efavirenz
600 mg Once Daily +
Lamivudine/Zidovudine
(n = 361)
%Blood and Lymphatic System Disorders Anemias NEC 8 5 Neutropenias 4 3 Ear and Labyrinth Disorders Ear disorders NEC 3 2 Gastrointestinal Disorders Flatulence, bloating, and distention 10 7 Gastrointestinal atonic and hypomotility disorders NEC 9 5 Gastrointestinal signs and symptoms NEC 3 2 General Disorders and Administration Site Conditions Body temperature perception 3 1 Infections and Infestations Upper respiratory tract infection 32 30 Bronchitis 13 9 Herpes infection 7 6 Bacterial infections NEC 6 3 Herpes zoster/varicella 5 4 Tinea infections 4 3 Lower respiratory tract and lung
infections3 2 Neisseria infections 3 0 Viral infections NEC 3 2 Musculoskeletal and Connective Tissue Disorders Joint-related signs and symptoms 6 5 Nervous System Disorders Paresthesias and dysesthesias 4 3 Memory loss (excluding dementia) 3 1 Renal and Urinary Disorders Bladder and urethral symptoms 4 3 Reproductive System and Breast Disorders Erection and ejaculation conditions and disorders 3 2 Respiratory, Thoracic, and Mediastinal Disorders Upper respiratory tract signs and symptoms 9 5 Skin and Subcutaneous Disorders Nail and nail bed conditions
(excluding infections and
infestations)6 2 Lipodystrophies 4 3 Acnes 3 2 Alopecias 2 1 Laboratory Abnormalities:
Table 8. Maximum Shift in Laboratory Test Values (without Regard to Baseline) ≥2% of Grade 3-4 Abnormalities (ACTG Criteria) in Trial A4001026 (96 Weeks) - * n = Total number of subjects evaluable for laboratory abnormalities. Percentages based on total subjects evaluated for each laboratory parameter. If the same subject in a given treatment group had greater than 1 occurrence of the same abnormality, only the most severe is counted.
Laboratory Parameter
Preferred Term
Limit Maraviroc
300 mg Twice Daily + Lamivudine/Zidovudine
(n = 353) *
%Efavirenz
600 mg Once Daily+ Lamivudine/Zidovudine
(n = 350) a
%Aspartate aminotransferase >5.0 x ULN 4.0 4.0 Alanine aminotransferase >5.0 x ULN 3.9 4.0 Creatine kinase >10.0 x ULN 3.9 4.8 Amylase >2.0 x ULN 4.3 6.0 Absolute neutrophil count <750/mm3 5.7 4.9 Hemoglobin <7.0 g/dL 2.9 2.3 ULN = Upper limit of normal.
Less Common Adverse Events in Clinical Trials:The following adverse events occurred in less than 2% of subjects treated with Maraviroc or at a rate similar to the comparator. These events have been included because of their seriousness and either increased frequency on Maraviroc or are potential risks due to the mechanism of action. Events attributed to the subjects underlying HIV-1 infection are not listed.
Blood and Lymphatic System:Marrow depression and hypoplastic anemia.
Cardiac Disorders:Unstable angina, acute cardiac failure, coronary artery disease, coronary artery occlusion, myocardial infarction, myocardial ischemia.
Hepatobiliary Disorders:Hepatic cirrhosis, hepatic failure, cholestatic jaundice, portal vein thrombosis, jaundice.
Infections and Infestations:Endocarditis, infective myositis, viral meningitis, pneumonia, treponema infections, septic shock, Clostridium difficile colitis, meningitis.
Musculoskeletal and Connective Tissue Disorders:Myositis, osteonecrosis, rhabdomyolysis, blood creatine kinase increased.
Neoplasms Benign, Malignant, and Unspecified (Including Cysts and Polyps):Abdominal neoplasm, anal cancer, basal cell carcinoma, Bowens disease, cholangiocarcinoma, diffuse large B-cell lymphoma, lymphoma, metastases to liver, esophageal carcinoma, nasopharyngeal carcinoma, squamous cell carcinoma, squamous cell carcinoma of skin, tongue neoplasm (malignant stage unspecified), anaplastic large cell lymphomas T- and null-cell types, bile duct neoplasms malignant, endocrine neoplasms malignant and unspecified.
Nervous System Disorders:Cerebrovascular accident, convulsions and epilepsy, tremor (excluding congenital), facial palsy, hemianopia, loss of consciousness, visual field defect.
Clinical Trials Experience in Pediatric Subjects
HIV-1Infected Pediatric Subjects:Trial A4001031 is an open-label trial in which 103 treatment-experienced, CCR5-tropic, HIV-1infected pediatric subjects aged 2 to less than 18 years weighing at least 10 kg received Maraviroc twice daily in combination with OBT. The dose of Maraviroc was based on body surface area (BSA) and on whether the subject was receiving potent CYP3A inhibitors and/or inducers. The median duration of therapy with Maraviroc was 131 weeks with 72% of subjects receiving study treatment for greater than 48 weeks and 62% of subjects receiving study treatment for 96 weeks.
In these 103 children and adolescents, the safety profile through 96 weeks was similar to that for adults. Most of the adverse reactions reported were mild to moderate; severe (Grade 3 and 4) adverse reactions occurred in 2% of subjects. The most common adverse reactions (all grades) reported with twice-daily therapy with Maraviroc were vomiting (12%), abdominal pain (4%), diarrhea (4%), nausea (4%), and dizziness (3%). Three subjects (3%) discontinued due to adverse events.
Maraviroc-related gastrointestinal adverse events through 48 weeks (nausea, vomiting, diarrhea, constipation, and abdominal pain/cramps) were observed more commonly in subjects who received the Maraviroc oral solution (21%) compared with those who received Maraviroc tablets (16%). Subjects were permitted to change formulations after Week 48.
6.2 Postmarketing Experience
The following adverse events have been identified during post-approval use of Maraviroc. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders
Stevens‑Johnson syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), toxic epidermal necrolysis (TEN).
-
7 DRUG INTERACTIONS
7.1 Effect of Concomitant Drugs on the Pharmacokinetics of Maraviroc
Maraviroc is metabolized by CYP3A and is also a substrate for P-glycoprotein (P-gp), organic anion-transporting polypeptide (OATP)1B1, and multidrug resistance-associated protein (MRP)2. The pharmacokinetics of maraviroc are likely to be modulated by inhibitors and inducers of CYP3A and P-gp and may be modulated by inhibitors of OATP1B1 and MRP2. Therefore, a dosage adjustment may be required when maraviroc is coadministered with those drugs [see Dosage and Administration ( 2.3, 2.4)] .
Concomitant use of maraviroc and St. John's wort ( Hypericum perforatum) or products containing St. John's wort is not recommended. Coadministration of maraviroc with St. John's wort is expected to substantially decrease maraviroc concentrations and may result in suboptimal levels of maraviroc and lead to loss of virologic response and possible resistance to maraviroc.
Additional drug interaction information is available [see Clinical Pharmacology (12.3)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Maraviroc during pregnancy. Physicians are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Limited data on the use of Maraviroc during pregnancy from the APR and case reports are not sufficient to inform a drug-associated risk of birth defects and miscarriage. In animal reproduction studies, no evidence of adverse developmental outcomes was observed with maraviroc. During organogenesis in the rat and rabbit, systemic exposures (AUC) to maraviroc were approximately 20 times (rats) and 5 times (rabbits) the exposure in humans at the recommended 300-mg twice-daily dose.
In the rat pre- and post-natal development study, maternal systemic exposure (AUC) to maraviroc was approximately 14 times the exposure in humans at the recommended 300-mg twice- daily dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data:Maraviroc was administered orally to pregnant rats (up to 1,000 mg per kg per day) and rabbits (up to 75 mg per kg per day) on gestation Days 6 to 17 and 7 to 19, respectively. No adverse effects on embryo-fetal development were observed at these dose levels, resulting in exposures (AUC) approximately 20 times (rats) and 5 times (rabbits) higher than human exposures at the recommended daily dose. In the rat pre- and post-natal development study, maraviroc was administered orally at up to 1,000 mg per kg per day on gestation Day 6 to lactation/post-partum Day 20, with development of the offspring (including fertility and reproductive performance) unaffected by maternal administration of maraviroc at an exposure (AUC) approximately 14 times higher than human exposure at the recommended daily dose.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV‑1–infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV‑1 infection.
There are no data on the presence of maraviroc in human milk, the effects on the breastfed infant, or the effects on milk production. When administered to lactating rats, maraviroc was present in milk (see Data). Because of the potential for (1) HIV transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants), and (3) serious adverse reactions in a breastfed infant similar to those seen in adults, instruct mothers not to breastfeed if they are receiving Maraviroc.
Data
Maraviroc (and related metabolites) was excreted into the milk of lactating rats following a single oral dose of maraviroc (100 mg per kg) on lactation Day 12, with a maximal milk concentration achieved one hour post-administration at a milk concentration approximately 2.5 times that of maternal plasma concentrations.
8.4 Pediatric Use
The safety and efficacy of maraviroc have been established in pediatric patients aged from aged 2 to less than 18 years. The use of Maraviroc in pediatric patients was supported by pharmacokinetic and safety data described below and by previous demonstration of efficacy in adult patients [see Indications and Usage (1), Dosage and Administration (2.4)].
HIV-1–Infected Pediatric Patients Aged 2 to Less Than 18 Years: The safety, pharmacokinetic profile, and antiviral activity of Maraviroc were evaluated in treatment- experienced, CCR5-tropic, HIV-1–infected pediatric subjects aged 2 to less than 18 years weighing at least 10 kg in an open-label, multicenter clinical trial, A4001031 [see Adverse Reactions (6.1), Clinical Studies (14.2)]. Pharmacokinetics were evaluated in a total of 98 pediatric subjects: 85 subjects received Maraviroc and concomitant medications that included potent CYP3A inhibitors with or without potent CYP3A inducers, 10 subjects received Maraviroc and noninteracting medications (not containing potent CYP3A inhibitors or potent CYP3A inducers), and three subjects received Maraviroc and medications that included potent CYP3A inducers without potent CYP3A inhibitors [see Clinical Pharmacology (12.3)].
There are insufficient data to make dosing recommendations for use of Maraviroc in pediatric patients concomitantly receiving potent CYP3A inhibitors and weighing less than 10 kg, or in any pediatric patients concomitantly receiving potent CYP3A inducers without a potent CYP3A inhibitor [see Dosage and Administration ( 2.4, 2.5)] .
Maraviroc is not recommended in pediatric patients weighing less than 10 kg.
8.5 Geriatric Use
There were insufficient numbers of subjects aged 65 and over in the clinical trials to determine whether they respond differently from younger subjects. In general, caution should be exercised when administering Maraviroc in elderly patients, also reflecting the greater frequency of decreased hepatic and renal function, of concomitant disease and other drug therapies
8.6 Renal Impairment
Recommended doses of Maraviroc for adult patients with impaired renal function (CrCl less than or equal to 80 mL per minute) are based on the results of a pharmacokinetic trial conducted in healthy adult subjects with various degrees of renal impairment. Maraviroc has not been studied in pediatric patients with renal impairment. There are no data to recommend specific doses of Maraviroc in pediatric patients with mild to moderate renal impairment [see Use in Specific Populations (8.4)] . Maraviroc is contraindicated in pediatric patients with severe renal impairment or ESRD on regular hemodialysis who are receiving potent CYP3A inhibitors [see Contraindications (4)] .
The pharmacokinetics of maraviroc in adult subjects with mild and moderate renal impairment was similar to that in subjects with normal renal function [see Clinical Pharmacology (12.3)] . A limited number of adult subjects with mild and moderate renal impairment in the Phase 3 clinical trials (n = 131 and n = 12, respectively) received the same dose of Maraviroc as that administered to subjects with normal renal function. In these subjects, there was no apparent difference in the adverse event profile for maraviroc compared with subjects with normal renal function.
If adult patients with severe renal impairment or ESRD not receiving a concomitant potent CYP3A inhibitor or inducer experience any symptoms of postural hypotension while taking Maraviroc 300 mg twice daily, the dose should be reduced to 150 mg twice daily. No trials have been performed in subjects with severe renal impairment or ESRD co-treated with potent CYP3A inhibitors or inducers. Hence, no dose of Maraviroc can be recommended, and Maraviroc is contraindicated for these patients [see Dosage and Administration (2.3), Contraindications (4), Warningsand Precautions (5.3), Clinical Pharmacology (12.3)] .
8.7 Hepatic Impairment
Maraviroc is principally metabolized by the liver; therefore, when administering this drug to patients with hepatic impairment, maraviroc concentrations may be increased. Maraviroc concentrations are higher when Maraviroc 150 mg is administered with a potent CYP3A inhibitor compared with following administration of 300 mg without a CYP3A inhibitor, so patients with moderate hepatic impairment who receive Maraviroc 150 mg with a potent CYP3A inhibitor should be monitored closely for maraviroc- associated adverse events. Maraviroc has not been studied in subjects with severe hepatic impairment or in pediatric patients with any degree of hepatic impairment [see Warnings and Precautions(5.1), Clinical Pharmacology (12.3)] .
-
10 OVERDOSAGE
The highest single dose administered in clinical trials was 1,200 mg. The dose-limiting adverse event was postural hypotension, which was observed at 600 mg. While the recommended dose for Maraviroc in patients receiving a CYP3A inducer without a CYP3A inhibitor is 600 mg twice daily, this dose is appropriate due to enhanced metabolism.
Prolongation of the QT interval was seen in dogs and monkeys at plasma concentrations 6 and 12 times, respectively, those expected in humans at the intended exposure of 300-mg equivalents twice daily. However, no significant QT prolongation was seen in the trials in treatment-experienced subjects with HIV using the recommended doses of maraviroc, or in a specific pharmacokinetic trial to evaluate the potential of maraviroc to prolong the QT interval [see Clinical Pharmacology (12.2)] .
There is no specific antidote for overdose with maraviroc. Treatment of overdose should consist of general supportive measures including keeping the patient in a supine position, careful assessment of patient vital signs, blood pressure, and electrocardiogram.
Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Hemodialysis had a minimal effect on maraviroc clearance and exposure in a trial in subjects with ESRD [see Clinical Pharmacology (12.3)] .
-
11 DESCRIPTION
Maraviroc is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120. Blocking this interaction prevents CCR5-tropic HIV-1 entry into cells.
Maraviroc film-coated tablets for oral administration contain 150, or 300 mg of maraviroc and the following inactive ingredients: dibasic calcium phosphate (anhydrous), magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The film coat (Opadry II Blue [85G20583]) contains FD&C blue # 2 aluminum lake, soya lecithin, polyethylene glycol (macrogol 3350), polyvinyl alcohol, talc, and titanium dioxide.
Maraviroc is chemically described as 4,4-difluoro-N-{(1S)-3-[exo-3-(3-isopropyl-5- methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1- phenylpropyl}cyclohexanecarboxamide.
The molecular formula is C29H41F2N5O and the structural formula is:
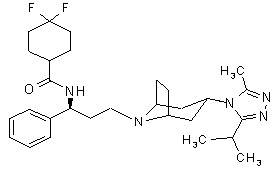
Maraviroc is a white to off-white powder with a molecular weight of 513.68. It is very soluble in methanol and is highly soluble across the physiological pH range (pH 1.0 to 7.5).
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Exposure-Response Relationship in Treatment-Experienced Adult Subjects
The relationship between maraviroc, modeled plasma trough concentration (C min) (1 to 9 samples per subject taken on up to 7 visits), and virologic response was evaluated in 973 treatment-experienced HIV-1-infected subjects with varied optimized background antiretroviral regimens in Trials A4001027 and A4001028. The C min, baseline viral load, baseline CD4+ cell count, and overall sensitivity score (OSS) were found to be important predictors of virologic success (defined as viral load less than 400 copies per mL at 24 weeks). Table 9 illustrates the proportions of subjects with virologic success (%) within each C minquartile for 150-mg twice-daily and 300-mg twice-daily groups.
Table 9. Treatment-Experienced Subjects with Virologic Success by C minQuartile (Q1-Q4) 150 mg Twice Daily
(with CYP3A Inhibitors)300 mg Twice Daily
(without CYP3A Inhibitors)n Median
Cmin% Subjects with Virologic Success
n Median
Cmin% Subjects with Virologic Success
Placebo 160 - 30.6 35 - 28.6 Q1 78 33 52.6 22 13 50.0 Q2 77 87 63.6 22 29 68.2 Q3 78 166 78.2 22 46 63.6 Q4 78 279 74.4 22 97 68.2 Exposure-Response Relationship in Treatment-Naive Adult Subjects
The relationship between maraviroc, modeled plasma trough concentration (C min) (1 to 12 samples per subject taken on up to 8 visits), and virologic response was evaluated in 294 treatment-naive HIV1-infected subjects receiving maraviroc 300 mg twice daily in combination with lamivudine/zidovudine in Trial A4001026. Table 10 illustrates the proportion (%) of subjects with virologic success less than 50 copies per mL at 48 weeks within each C minquartile for the 300-mg twice-daily dose.
Table 10. Treatment-Naive Subjects with Virologic Success by C minQuartile (Q1-Q4) 300 mg Twice Daily n Median C min % Subjects with Virologic Success
Q1 75 23 57.3 Q2 72 39 72.2 Q3 73 56 74.0 Q4 74 81 83.8 Eighteen of 75 (24%) subjects in Q1 had no measurable maraviroc concentration on at least one occasion versus 1 of 73 and 1 of 74 in Q3 and Q4, respectively.
Effects on Electrocardiogram
A placebo-controlled, randomized, crossover trial to evaluate the effect on the QT interval of healthy male and female volunteers was conducted with 3 single oral doses of maraviroc and moxifloxacin. The placebo-adjusted mean maximum (upper 1-sided 95% CI) increases in QTc from baseline after 100, 300, and 900 mg of maraviroc were –2 (0), -1 (1), and 1 (3) msec, respectively, and 13 (15) msec for moxifloxacin 400 mg. No subject in any group had an increase in QTc of greater than or equal to 60 msec from baseline. No subject experienced an interval exceeding the potentially clinically relevant threshold of 500 msec.
12.3 Pharmacokinetics
Table 11. Mean Maraviroc Pharmacokinetic Parameters in Adults *The estimated exposure is lower compared with other trials possibly due to sparse sampling, food effect, compliance, and concomitant medications.
Patient Population Maraviroc Dose n AUC12
(ng.h/mL)Cmax (ng/mL) Cmin (ng/mL) Healthy volunteers (Phase 1) 300 mg twice daily 64 2908 888 43.1 Asymptomatic HIV subjects (Phase 2a) 300 mg twice daily 8 2550 618 33.6 Treatment- experienced HIV subjects (Phase 3)* 300 mg twice daily 94 1513 266 37.2 150 mg twice daily
(+ CYP3A inhibitor)375 2463 332 101 Treatment- naive HIV Subjects (Phase 2b/3)* 300 mg twice daily 344 1865 287 60 Absorption
Peak maraviroc plasma concentrations are attained 0.5 to 4 hours following single oral doses of 1 to 1,200 mg administered to uninfected volunteers. The pharmacokinetics of oral maraviroc are not dose proportional over the dose range.
The absolute bioavailability of a 100‑mg dose is 23% and is predicted to be 33% at 300 mg. Maraviroc is a substrate for the efflux transporter P-gp.
Effect of Food on Oral Absorption:Coadministration of a 300‑mg tablet with a high‑fat breakfast reduced maraviroc C max and AUC by 33% and coadministration of 75 mg of oral solution with a high-fat breakfast reduced maraviroc AUC by 73% in healthy adult volunteers. Studies with the tablet formulation demonstrated a reduced food effect at higher doses.
There were no food restrictions in the adult trials (using the tablet formulation) or in the pediatric trial (using both tablet and oral solution formulations) that demonstrated the efficacy/antiviral activity and safety of maraviroc [see Clinical Studies ( 14.1, 14.2)] .
Distribution
Maraviroc is bound (approximately 76%) to human plasma proteins, and shows moderate affinity for albumin and alpha‑1 acid glycoprotein. The volume of distribution of maraviroc is approximately 194 L.
Elimination
Metabolism:Trials in humans and in vitro studies using human liver microsomes and expressed enzymes have demonstrated that maraviroc is principally metabolized by the cytochrome P450 system to metabolites that are essentially inactive against HIV‑1. In vitro studies indicate that CYP3A is the major enzyme responsible for maraviroc metabolism. In vitro studies also indicate that polymorphic enzymes CYP2C9, CYP2D6, and CYP2C19 do not contribute significantly to the metabolism of maraviroc.
Maraviroc is the major circulating component (~42% drug‑related radioactivity) following a single oral dose of 300 mg [ 14C]-maraviroc. The most significant circulating metabolite in humans is a secondary amine (~22% radioactivity) formed by N‑dealkylation. This polar metabolite has no significant pharmacological activity. Other metabolites are products of mono‑oxidation and are only minor components of plasma drug‑related radioactivity.
Excretion:The terminal half‑life of maraviroc following oral dosing to steady state in healthy subjects was 14 to 18 hours. A mass balance/excretion trial was conducted using a single 300‑mg dose of 14C-labeled maraviroc. Approximately 20% of the radiolabel was recovered in the urine and 76% was recovered in the feces over 168 hours. Maraviroc was the major component present in urine (mean of 8% dose) and feces (mean of 25% dose). The remainder was excreted as metabolites.
Specific Populations
Patients with Hepatic Impairment:Maraviroc is primarily metabolized and eliminated by the liver. A trial compared the pharmacokinetics of a single 300‑mg dose of Maraviroc in subjects with mild (Child‑Pugh Class A, n = 8) and moderate (Child‑Pugh Class B, n = 8) hepatic impairment with pharmacokinetics in healthy subjects (n = 8). The mean C max and AUC were 11% and 25% higher, respectively, for subjects with mild hepatic impairment, and 32% and 46% higher, respectively, for subjects with moderate hepatic impairment compared with subjects with normal hepatic function. These changes do not warrant a dose adjustment. Maraviroc concentrations are higher when Maraviroc, 150 mg is administered with a potent CYP3A inhibitor compared with following administration of 300 mg without a CYP3A inhibitor, so patients with moderate hepatic impairment who receive Maraviroc, 150 mg with a potent CYP3A inhibitor should be monitored closely for maraviroc‑associated adverse events. The pharmacokinetics of maraviroc have not been studied in subjects with severe hepatic impairment [see Warnings and Precautions (5.1)] .
Patients with Renal Impairment:A trial compared the pharmacokinetics of a single 300‑mg dose of Maraviroc in adult subjects with severe renal impairment (CrCl less than 30 mL per minute, n = 6) and ESRD (n = 6) with healthy volunteers (n = 6). Geometric mean ratios for maraviroc C maxand AUC infwere 2.4‑fold and 3.2‑fold higher, respectively, for subjects with severe renal impairment, and 1.7‑fold and 2.0‑fold higher, respectively, for subjects with ESRD as compared with subjects with normal renal function in this trial. Hemodialysis had a minimal effect on maraviroc clearance and exposure in subjects with ESRD. Exposures observed in subjects with severe renal impairment and ESRD were within the range observed in previous 300‑mg single‑dose trials of Maraviroc in healthy volunteers with normal renal function. However, maraviroc exposures in the subjects with normal renal function in this trial were 50% lower than those observed in previous trials. Based on the results of this trial, no dose adjustment is recommended for patients with renal impairment receiving Maraviroc without a potent CYP3A inhibitor or inducer. However, if patients with severe renal impairment or ESRD experience any symptoms of postural hypotension while taking Maraviroc, 300 mg twice daily, their dose should be reduced to 150 mg twice daily [see Dosage and Administration (2.3), Warnings and Precautions (5.3)] .
In addition, the trial compared the pharmacokinetics of multiple‑dose Maraviroc in combination with saquinavir/ritonavir 1,000/100 mg twice daily (a potent CYP3A inhibitor combination) for 7 days in subjects with mild renal impairment (CrCl greater than 50 and less than or equal to 80 mL per minute, n = 6) and moderate renal impairment (CrCl greater than or equal to 30 and less than or equal to 50 mL per minute, n = 6) with healthy volunteers with normal renal function (n = 6). Subjects received 150 mg of Maraviroc at different dose frequencies (healthy volunteers every 12 hours; mild renal impairment every 24 hours; moderate renal impairment every 48 hours). Compared with healthy volunteers (dosed every 12 hours), geometric mean ratios for maraviroc AUC tau, C max, and C min were 50% higher, 20% higher, and 43% lower, respectively, for subjects with mild renal impairment (dosed every 24 hours). Geometric mean ratios for maraviroc AUC tau, C max, and C min were 16% higher, 29% lower, and 85% lower, respectively, for subjects with moderate renal impairment (dosed every 48 hours) compared with healthy volunteers (dosed every 12 hours). Based on the data from this trial, no adjustment in dose is recommended for patients with mild or moderate renal impairment [see Dosage and Administration (2.3)] .
Pediatric Patients: Aged 2 to Less Than 18 Years:The pharmacokinetics of maraviroc were evaluated in CCR5-tropic, HIV-1infected, treatment-experienced pediatric subjects aged 2 to less than 18 years. In the dose-finding stage of Trial A4001031, doses were administered with food on intensive pharmacokinetic evaluation days and optimized to achieve an average concentration over the dosing interval (C avg) of greater than 100 ng per mL. Throughout the trial, on non-intensive pharmacokinetic evaluation days maraviroc was taken with or without food. The initial dose of maraviroc was based on BSA and concomitant medication category (i.e., presence of CYP3A inhibitors and/or inducers). The conversion of dosing to a weight (kg)-band basis in children provides comparable exposures with those observed in the trial at the corresponding BSA.
Maraviroc pharmacokinetic parameters in pediatric subjects aged 2 to less than 18 years receiving potent CYP3A inhibitors with or without a potent CYP3A inducer were similar to those observed in adults (Table 12).
Table 12. Maraviroc Pharmacokinetic Parameters in Treatment-Experienced Pediatric Patients Receiving Maraviroc with Potent CYP3A Inhibitors (with or without a Potent CYP3A Inducer) *Model-predicted steady-state pharmacokinetic parameters are presented.
Weight Dose of Maraviroc Maraviroc Pharmacokinetic Parameter* Geometric Mean AUC12
(ng.h/mL)Cavg (ng/mL) Cmax (ng/mL) Cmin (ng/mL) 10 kg to
<20 kg50 mg twice daily 2,349 196 324 78 20 kg to
<30 kg75 mg twice daily 3,020 252 394 118 30 kg to
<40 kg100 mg twice daily 3,229 269 430 126 40 kg 150 mg twice daily 4,044 337 563 152 Clinical pharmacokinetic data in pediatric patients aged 2 to less than 18 years receiving noninteracting concomitant medications are limited. Based on population pharmacokinetic modeling and simulation, the recommended dosing regimen of Maraviroc for this population is predicted to result in similar maraviroc exposures when compared with exposures achieved in adults receiving Maraviroc, 300 mg twice daily (with noninteracting concomitant medications) [see Dosage and Administration (2.4)] .
Geriatric Patients:Pharmacokinetics of maraviroc have not been fully evaluated in the elderly (aged 65 years and older). Based on population pharmacokinetic analyses, age did not have a clinically relevant effect on maraviroc exposure in subjects up to age 65 years [see Use in SpecificPopulations (8.5)] .
Race and Gender:Based on population pharmacokinetics and 2 clinical CYP3A5 genotype analyses for race, no dosage adjustment is recommended based on race or gender.
Drug Interaction Studies
Effect of Concomitant Drugs on the Pharmacokinetics of Maraviroc:Maraviroc is a substrate of CYP3A and P-gp and hence its pharmacokinetics are likely to be modulated by inhibitors and inducers of these enzymes/transporters. The CYP3A/P-gp inhibitors ketoconazole, lopinavir/ritonavir, ritonavir, darunavir/ritonavir, saquinavir/ritonavir, and atazanavir ± ritonavir all increased the C maxand AUC of maraviroc (Table 14). The CYP3A and/or P-gp inducers rifampin, etravirine, and efavirenz decreased the C maxand AUC of maraviroc (Table 14). While not studied, potent CYP3A and/or P-gp inducers carbamazepine, phenobarbital, and phenytoin are expected to decrease maraviroc concentrations. Based on in vitro study results, maraviroc is also a substrate of OATP1B1 and MRP2; its pharmacokinetics may be modulated by inhibitors of these transporters.
Tipranavir/ritonavir (net CYP3A inhibitor/P-gp inducer) did not affect the steady‑state pharmacokinetics of maraviroc (Table 14). Cotrimoxazole and tenofovir did not affect the pharmacokinetics of maraviroc.100 mg b.i.d.300 mg b.i.d
Table 14. Effect of Coadministered Agents on the Pharmacokinetics of Maraviroc *Compared with historical data.
Coadministered Drug
and Dosen Dose of Maraviroc Ratio (90% CI) of Maraviroc Pharmacokinetic Parameters with/without Coadministered Drug (No Effect = 1.00) Cmin AUCtau Cmax CYP3A and/or P-gp Inhibitors Ketoconazole400 mg q.d. 12 100 mg b.i.d. 3.75
(3.01, 4.69)
5.00
(3.98, 6.29)
3.38
(2.38, 4.78)
Ritonavir
100 mg b.i.d.
8 100 mg b.i.d. 4.55
(3.37, 6.13)
2.61
(1.92, 3.56)
1.28
(0.79, 2.09)
Saquinavir (soft gel
capsules) /ritonavir
1,000 mg/100 mg b.i.d.11 100 mg b.i.d. 11.3
(8.96, 14.1)9.77
(7.87, 12.14)
4.78
(3.41, 6.71)Lopinavir/ritonavir400 mg/100 mg b.i.d. 11 300 mg b.i.d. 9.24
(7.98, 10.7)
3.95
(3.43, 4.56)
1.97
(1.66, 2.34)
Atazanavir
400 mg q.d.
12 300 mg b.i.d. 4.19
(3.65, 4.80)
3.57
(3.30, 3.87)
2.09
(1.72, 2.55)
Atazanavir/ritonavir
300 mg/100 mg q.d.
12 300 mg b.i.d. 6.67
(5.78, 7.70)
4.88
(4.40, 5.41)
2.67
(2.32, 3.08)
Darunavir/ritonavir
600 mg/100 mg b.i.d.
12 150 mg b.i.d. 8.00
(6.35, 10.1)
4.05
(2.94, 5.59)
2.29
(1.46, 3.59)
Elvitegravir/ritonavir
150 mg/100 mg q.d.
11 150 mg b.i.d. 4.23
(3.47, 5.16)
2.86
(2.33, 3.51)
2.15
(1.71, 2.69)
CYP3A and/or P-gp Inducers Efavirenz
600 mg q.d.
12 100 mg b.i.d. 0.55
(0.43, 0.72)
0.55
(0.49, 0.62)
0.49
(0.38, 0.63)
Efavirenz
600 mg q.d.
12 200 mg b.i.d.
(+ efavirenz):100 mg b.i.d.
(alone)1.09
(0.89, 1.35)1.15
(0.98, 1.35)1.16
(0.87, 1.55)Rifampicin
600 mg q.d.
12 100 mg b.i.d. 0.22
(0.17, 0.28)
0.37
(0.33, 0.41)
0.34
(0.26, 0.43)
Rifampicin
600 mg q.d.12 200 mg b.i.d.
(+efavirenz):100 mg b.i.d.
(alone)
0.66
(0.54, 0.82)1.04
(0.89, 1.22)0.97
(0.72, 1.29)Etravirine
200 mg b.i.d.
14 300 mg b.i.d
0.61
(0.53, 0.71)
0.47
(0.38, 0.58)
0.40
(0.28, 0.57)
Nevirapine*
200 mg b.i.d.
(+ lamivudine 150 mg
b.i.d., tenofovir 300 mg
q.d.)
8 300 mg
single dose- 1.01
(0.65, 1.55)1.54
(0.94, 2.51)CYP3A and/or P-gp Inhibitors and Inducers Lopinavir/ritonavir +
efavirenz
400 mg/100 mg b.i.d.
+ 600 mg q.d.
11 300 mg b.i.d. 6.29
(4.72, 8.39)2.53
(2.24, 2.87)1.25
(1.01, 1.55)Saquinavir (soft gel
capsules) /ritonavir +
efavirenz
1,000 mg/100 mg b.i.d.
11 100 mg b.i.d. 8.42
(6.46, 10.97)5.00
(4.26, 5.87)2.26
(1.64, 3.11)Darunavir/ritonavir +
etravirine
600 mg/100 mg b.i.d.
+ 200 mg b.i.d.
10 150 mg b.i.d. 5.27
(4.51, 6.15)3.10
(2.57, 3.74)1.77
(1.20, 2.60)Fosamprenavir/ritonavir
700 mg/100 mg b.i.d.
14 300 mg b.i.d. 4.74
(4.03, 5.57)2.49
(2.19, 2.82)1.52
(1.27, 1.82)Fosamprenavir/ritonavir
1,400 mg/100 mg q.d.
14 300 mg q.d. 1.80
(1.53, 2.13)2.26
(1.99, 2.58)1.45
(1.20, 1.74)Tipranavir/ritonavir
500 mg/200 mg b.i.d.
12 150 mg b.i.d. 1.80
(1.55, 2.09)1.02
(0.85, 1.23)0.86
(0.61, 1.21)Other Raltegravir
400 mg b.i.d.
17 300 mg b.i.d. 0.90
(0.85, 0.96)0.86
(0.80, 0.92)0.79
(0.67, 0.94)Effect of Maraviroc on the Pharmacokinetics of Concomitant Drugs:Maraviroc is unlikely to inhibit the metabolism of coadministered drugs metabolized by the following cytochrome P enzymes (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP3A) or to inhibit the uptake of OATP1B1 or the export of MRP2 because maraviroc did not inhibit activity of those enzymes or transporters at clinically relevant concentrations in vitro. Maraviroc does not induce CYP1A2 in vitro. Additionally, in vitro studies have shown that maraviroc is not a substrate for, and does not inhibit, any of the major renal uptake inhibitors (organic anion transporter [OAT]1, OAT3, organic cation transporter [OCT]2, novel organic cation transporter [OCTN]1, and OCTN2) at clinically relevant concentrations.
In vitro results suggest that maraviroc could inhibit P-gp in the gut. However, maraviroc did not significantly affect the pharmacokinetics of digoxin in vivo, indicating maraviroc may not significantly inhibit or induce P-gp clinically.
Drug interaction trials were performed with maraviroc and other drugs likely to be coadministered or commonly used as probes for pharmacokinetic interactions (Table 14).
Coadministration of fosamprenavir 700 mg/ritonavir 100 mg twice daily and maraviroc 300 mg twice daily decreased the Cmin and AUC of amprenavir by 36% and 35%, respectively. Coadministration of fosamprenavir 1,400 mg/ritonavir 100 mg once daily and maraviroc 300 mg once daily decreased the Cmin and AUC of amprenavir by 15% and 30%, respectively. No dosage adjustment is necessary when Maraviroc is dosed 150 mg twice daily in combination with fosamprenavir/ritonavir dosed once or twice daily. Fosamprenavir should be given with ritonavir when coadministered with Maraviroc.
Maraviroc had no significant effect on the pharmacokinetics of elvitegravir, zidovudine, or lamivudine. Maraviroc decreased the Cmin and AUC of raltegravir by 27% and 37%, respectively, which is not clinically significant. Maraviroc had no clinically relevant effect on the pharmacokinetics of midazolam, the oral contraceptives ethinylestradiol and levonorgestrel, no effect on the urinary 6-hydroxycortisol/cortisol ratio, suggesting no induction of CYP3A in vivo. Maraviroc had no effect on the debrisoquine metabolic ratio (MR) at 300 mg twice daily or less in vivo and did not cause inhibition of CYP2D6 in vitro until concentrations greater than 100 microM. However, there was 234% increase in debrisoquine MR on treatment compared with baseline at 600 mg once daily, suggesting potential inhibition of CYP2D6 at higher doses.
12.4 Microbiology
Mechanism of Action
Maraviroc is a member of a therapeutic class called CCR5 co-receptor antagonists. Maraviroc selectively binds to the human chemokine receptor CCR5 present on the cell membrane, preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5- tropic HIV-1 to enter cells. CXCR4-tropic and dual-tropic HIV-1 entry is not inhibited by maraviroc.
Antiviral Activity in Cell Culture
Maraviroc inhibits the replication of CCR5-tropic laboratory strains and primary isolates of HIV-1 in models of acute peripheral blood leukocyte infection. The mean EC 50value (50% effective concentration) for maraviroc against HIV-1 group M isolates (subtypes A to J and circulating recombinant form AE) and group O isolates ranged from 0.1 to 4.5 nM (0.05 to 2.3 ng per mL) in cell culture.
When used with other antiretroviral agents in cell culture, the combination of maraviroc was not antagonistic with non-nucleoside reverse transcriptase inhibitors (NNRTIs: efavirenz and nevirapine), NRTIs (abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zalcitabine, and zidovudine), or protease inhibitors (PIs: amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir). Maraviroc was not antagonistic with the HIV-1 gp41 fusion inhibitor enfuvirtide. Maraviroc was not active against CXCR4-tropic and dual-tropic viruses (EC 50value greater than 10 microM). The antiviral activity of maraviroc against HIV-2 has not been evaluated.
Resistance in Cell Culture:HIV-1 variants with reduced susceptibility to maraviroc have been selected in cell culture following serial passage of 2 CCR5-tropic viruses (CCl/85 and RU570). The maraviroc-resistant viruses remained CCR5-tropic with no evidence of a change from a CCR5-tropic virus to a CXCR4-using virus. Two amino acid residue substitutions in the V3-loop region of the HIV-1 envelope glycoprotein (gp160), A316T, and I323V (HXB2 numbering), were shown to be necessary for the maraviroc-resistant phenotype in the HIV-1 isolate CCl/85. In the RU570 isolate a 3-amino acid residue deletion in the V3 loop, QAI (HXB2 positions 315 to 317), was associated with maraviroc resistance. The relevance of the specific gp120 substitutions observed in maraviroc-resistant isolates selected in cell culture to clinical maraviroc resistance is not known. Maraviroc-resistant viruses were characterized phenotypically by concentration- response curves that did not reach 100% inhibition in phenotypic drug assays, rather than increases in EC 50values.
Cross-Resistance in Cell Culture:Maraviroc had antiviral activity against HIV‑1 clinical isolates resistant to NNRTIs, NRTIs, PIs, and the gp41 fusion inhibitor enfuvirtide in cell culture (EC 50values ranged from 0.7 to 8.9 nM [0.36 to 4.57 ng per mL]). Maraviroc‑resistant viruses that emerged in cell culture remained susceptible to enfuvirtide and the protease inhibitor saquinavir.
Clinical Resistance:Virologic failure on maraviroc can result from genotypic and phenotypic resistance to maraviroc, through outgrowth of undetected CXCR4-using virus present before maraviroc treatment (see Tropismbelow), through resistance to background therapy drugs (Table 15), or due to low exposure to maraviroc [see Clinical Pharmacology (12.2)] .
Antiretroviral Treatment-Experienced Adult Subjects (Trials A4001027 and A4001028):Week 48 data from treatment-experienced subjects failing maraviroc-containing regimens with CCR5-tropic virus (n = 58) have identified 22 viruses that had decreased susceptibility to maraviroc characterized in phenotypic drug assays by concentration- response curves that did not reach 100% inhibition. Additionally, CCR5-tropic virus from 2 of these treatment-failure subjects had greater than or equal to 3-fold shifts in EC 50values for maraviroc at the time of failure.
Fifteen of these viruses were sequenced in the gp120 encoding region and multiple amino acid substitutions with unique patterns in the heterogeneous V3 loop region were detected. Changes at either amino acid position 308 or 323 (HXB2 numbering) were seen in the V3 loop in 7 of the subjects with decreased maraviroc susceptibility. Substitutions outside the V3 loop of gp120 may also contribute to reduced susceptibility to maraviroc.
Antiretroviral Treatment-Naive Adult Subjects (Trial A4001026):Treatment-naive subjects receiving Maraviroc had more virologic failures and more treatment-emergent resistance to the background regimen drugs compared with those receiving efavirenz (Table 15).
Table 15. Development of Resistance to Maraviroc or Efavirenz and Background Drugs in Antiretroviral Treatment-Naive Trial A4001026 for Patients with Only CCR5-Tropic Virus at Screening Using Enhanced Sensitivity TROFILE Assay Maraviroc Efavirenz Total N in dataset (as-treated) 273 241 Total virologic failures (as-treated) 85 (31%) 56 (23%) Evaluable virologic failures with post baseline genotypic and phenotypic data 73 43 Lamivudine resistance 39 (53%) 13 (30%) Zidovudine resistance 2 (3%) 0 Efavirenz resistance – 23 (53%) Phenotypic resistance to maraviroc a 19 (26%) – aIncludes subjects failing with CXCR4- or dual/mixed-tropism because these viruses are not intrinsically susceptible to maraviroc. In an as‑treated analysis of treatment‑naive subjects at 96 weeks, 32 subjects failed a maraviroc‑containing regimen with CCR5‑tropic virus and had a tropism result at failure; 7 of these subjects had evidence of maraviroc phenotypic resistance defined as concentration‑response curves that did not reach 95% inhibition. One additional subject had a greater than or equal to 3‑fold shift in the EC 50value for maraviroc at the time of failure. A clonal analysis of the V3 loop amino acid envelope sequences was performed from 6 of the 7 subjects. Changes in V3 loop amino acid sequence differed between each of these different subjects, even for those infected with the same virus clade, suggesting that there are multiple diverse pathways to maraviroc resistance. The subjects who failed with CCR5‑tropic virus and without a detectable maraviroc shift in susceptibility were not evaluated for genotypic resistance.
Of the 32 maraviroc virologic failures failing with CCR5‑tropic virus, 20 (63%) also had genotypic and/or phenotypic resistance to background drugs in the regimen (lamivudine, zidovudine).
Tropism:In both treatment‑experienced and treatment‑naive subjects, detection of CXCR4‑using virus prior to initiation of therapy has been associated with a reduced virologic response to maraviroc.
Antiretroviral Treatment‑Experienced Subjects (Trials A4001027 and A4001028):In the majority of cases, treatment failure on maraviroc was associated with detection of CXCR4‑using virus (i.e., CXCR4- or dual/mixed‑tropic) which was not detected by the tropism assay prior to treatment. CXCR4‑using virus was detected at failure in approximately 55% of subjects who failed treatment on maraviroc by Week 48, as compared with 9% of subjects who experienced treatment failure in the placebo arm. To investigate the likely origin of the on‑treatment CXCR4‑using virus, a detailed clonal analysis was conducted on virus from 20 representative subjects (16 subjects from the maraviroc arms and 4 subjects from the placebo arm) in whom CXCR4‑using virus was detected at treatment failure. From analysis of amino acid sequence differences and phylogenetic data, it was determined that CXCR4‑using virus in these subjects emerged from a low level of pre-existing CXCR4‑using virus not detected by the tropism assay (which is population-based) prior to treatment rather than from a co-receptor switch from CCR5‑tropic virus to CXCR4‑using virus resulting from mutation in the virus.
Detection of CXCR4‑using virus prior to initiation of therapy has been associated with a reduced virological response to maraviroc. Furthermore, subjects failing twice-daily maraviroc at Week 48 with CXCR4‑using virus had a lower median increase in CD4+ cell counts from baseline (+41 cells per mm 3) than those subjects failing with CCR5‑tropic virus (+162 cell s per mm 3). The median increase in CD4+ cell count in subjects failing in the placebo arm was +7 cell s per mm 3.
Antiretroviral Treatment‑Naive Subjects (Trial A4001026):In a 96‑week trial of antiretroviral treatment‑naive subjects, 14% (12 of 85) who had only CCR5‑tropic virus at screening with an enhanced sensitivity tropism assay (TROFILE) and failed therapy on maraviroc had CXCR4‑using virus at the time of treatment failure. A detailed clonal analysis was conducted in 2 previously antiretroviral treatment‑naive subjects enrolled in a Phase 2a monotherapy trial who had CXCR4‑using virus detected after 10 days’ treatment with maraviroc. Consistent with the detailed clonal analysis conducted in treatment‑experienced subjects, the CXCR4‑using variants appear to emerge from outgrowth of a pre‑existing undetected CXCR4‑using virus. Screening with an enhanced sensitivity tropism assay reduced the number of maraviroc virologic failures with CXCR4- or dual/mixed‑tropic virus at failure to 12 compared with 24 when screening with the original tropism assay. All but one (11 of 12; 92%) of the maraviroc failures failing with CXCR4- or dual/mixed‑tropic virus also had genotypic and phenotypic resistance to the background drug lamivudine at failure and 33% (4 of 12) developed zidovudine-associated resistance substitutions.
Subjects who had only CCR5‑tropic virus at baseline and failed maraviroc therapy with CXCR4‑using virus had a median increase in CD4+ cell counts from baseline of +113 cells per mm 3while those subjects failing with CCR5‑tropic virus had an increase of +135 cells per mm 3. The median increase in CD4+ cell count in subjects failing in the efavirenz arm was +95 cells per mm 3.
Antiretroviral Treatment‑Experienced Pediatric Subjects (Trial A4001031):In the Week 48 analysis of Trial A4001031 (n = 103), the mechanisms of resistance to maraviroc observed in the treatment-experienced pediatric population were similar to those observed in adult populations: reasons for virologic failure included failing with CXCR4- or dual/mixed-tropic virus, evidence of reduced maraviroc susceptibility as measured by a decrease in maximal percentage inhibition (MPI), and emergence of resistance to background drug in the regimen.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term oral carcinogenicity studies of maraviroc were carried out in rasH2 transgenic mice (6 months) and in rats for up to 96 weeks (females) and 104 weeks (males). No drug-related increases in tumor incidence were found in mice at 1,500 mg per kg per day and in male and female rats at 900 mg per kg per day. The highest exposures in rats were approximately 11 times those observed in humans at the therapeutic dose of 300 mg twice daily for the treatment of HIV-1 infection.
Mutagenesis
Maraviroc was not genotoxic in the reverse mutation bacterial test (Ames test in Salmonella and E. coli), a chromosome aberration test in human lymphocytes, and mouse bone marrow micronucleus test.
Impairment of Fertility
Maraviroc did not impair mating or fertility of male or female rats and did not affect sperm of treated male rats at approximately 20-fold higher exposures (AUC) than in humans given the recommended 300-mg twice-daily dose.
-
14 CLINICAL STUDIES
14.1 Clinical Studies in Adult Subjects
The clinical efficacy and safety of Maraviroc are derived from analyses of data from 3 trials in adult subjects infected with CCR5-tropic HIV-1: Trials A4001027 and A4001028 in antiretroviral treatment-experienced adult subjects and Trial A4001026 in treatment naive subjects. These trials were supported by a 48-week trial in antiretroviral treatment- experienced adult subjects infected with dual/mixed-tropic HIV-1, Trial A4001029.
Trials in CCR5-Tropic, Treatment-Experienced Subjects
Trials A4001027 and A4001028 were double-blind, randomized, placebo-controlled, multicenter trials in subjects infected with CCR5-tropic HIV-1. Subjects were required to have an HIV-1 RNA greater than 5,000 copies per mL despite at least 6 months of prior therapy with at least 1 agent from 3 of the 4 antiretroviral drug classes (greater than or equal to 1 NRTI, greater than or equal to 1 NNRTI, greater than or equal to 2 PIs, and/or enfuvirtide) or documented resistance to at least 1 member of each class. All subjects received an optimized background regimen consisting of 3 to 6 antiretroviral agents (excluding low-dose ritonavir) selected on the basis of the subjects prior treatment history and baseline genotypic and phenotypic viral resistance measurements. In addition to the optimized background regimen, subjects were then randomized in a 2:2:1 ratio to Maraviroc 300 mg once daily, Maraviroc 300 mg twice daily, or placebo. Doses were adjusted based on background therapy as described in Dosage and Administration (2), Table 1.
In the pooled analysis for Trials A4001027 and A4001028, the demographics and baseline characteristics of the treatment groups were comparable (Table 16). Of the 1,043 subjects with a CCR5 tropism result at screening, 7.6% had a dual/mixed-tropism result at the baseline visit 4 to 6 weeks later. This illustrates the background change from CCR5- to dual/mixed-tropism result over time in this treatment-experienced population, prior to a change in antiretroviral regimen or administration of a CCR5 co-receptor antagonist.
Table 16. Demographic and Baseline Characteristics of Subjects in Trials A4001027 and A4001028 Maraviroc Twice Daily (n = 426) Placebo (n = 209) Age (years)
Mean (range)46.3 (21-73) 45.7 (29-72) Sex:
Male
Female382 (89.7%)
44 (10.3%)185 (88.5%)
24 (11.5%)Race:
White
Black
Other363 (85.2%)
51 (12.0%)
12 (2.8%)178 (85.2%)
26 (12.4%)
5 (2.4%)Region:
U.S.
Non-U.S276 (64.8%)
150 (35.2%)135 (64.6%)
74 (35.4%)Subjects with previous enfuvirtide use 142 (33.3%) 62 (29.7%) Subjects with enfuvirtide as part of OBT 182 (42.7%) 91 (43.5%) Baseline plasma HIV-1 RNA (log10 copies/mL)
Mean (range)4.85 (2.96-6.88) 4.86 (3.46-7.07) Subjects with screening viral load >100,000 copies/mL 179 (42.0%) 84 (40.2%) Baseline CD4+ cell count (cells/mm3)
Median (range)167 (2-820) 171 (1-675) Subjects with baseline CD4+ cell count
200 cells/mm3)250 (58.7%) 118 (56.5%) Subjects with Overall Susceptibility Score (OSS):a 0 57 (13.4%) 35 (16.7%) 1 136 (31.9%) 44 (21.1%) 2 104 (24.4%) 59 (28.2%) 3 125 (29.3%) 66 (31.6%) Subjects with enfuvirtide resistance substitutions 90 (21.2%) 45 (21.5%) Median number of resistance-associated:b PI substitutions 10 10 NNRTI substitutions 1 1 NRTI substitutions 6 6 NNRTI = Non-nucleoside reverse transcriptase inhibitors; NRTI = nucleoside reverse transcriptase inhibitors; OBT = optimized background therapy; PI = protease inhibitor.
aOSS - Sum of active drugs in OBT based on combined information from genotypic and phenotypic testing.
bResistance substitutions based on IAS guidelines. 1
The Week 48 results for the pooled Trials A4001027 and A4001028 are shown in Table 17.
Table 17. Outcomes of Randomized Treatment at Week 48 in Trials A4001027 and A4001028 Outcome Maraviroc Twice Daily (n = 426) Placebo
(n = 209)Mean Difference Mean change from Baseline to Week 48 in HIV-1 RNA (log10 copies/mL) -1.84 -0.78 -1.05 <400 copies/mL at Week 48 239 (56%) 47 (22%) 34% <50 copies/mL at Week 48 194 (46%) 35 (17%) 29% Discontinuations:
Insufficientclinical responseAdverse events
Other
97 (23%)
19 (4%)
27 (6%)113 (54%)
11 (5%)
18 (9%)Subjects with treatment-emergent CDC Category C events 22 (5%) 16 (8%) Deaths (during trial or within 28 days of last dose) 9 (2%)* 1 (0.5%) *One additional subject died while receiving open-label therapy with Maraviroc subsequent to discontinuing double-blind placebo due to insufficient response. After 48 weeks of therapy, the proportions of subjects with HIV-1 RNA less than 400 copies per mL receiving Maraviroc compared with placebo were 56% and 22%, respectively. The mean changes in plasma HIV-1 RNA from baseline to Week 48 were 1.84 log10 copies per mL for subjects receiving Maraviroc + OBT compared with 0.78 log10 copies per mL for subjects receiving OBT only. The mean increase in CD4+ cell count was higher on Maraviroc twice daily + OBT (124 cells per mm 3) than on placebo + OBT (60 cells per mm 3).
Trial in Dual/Mixed-Tropic, Treatment-Experienced Subjects
Trial A4001029 was an exploratory, randomized, double-blind, multicenter trial to determine the safety and efficacy of Maraviroc in subjects infected with dual/mixed co- receptor tropic HIV-1. The inclusion/exclusion criteria were similar to those for Trials A4001027 and A4001028 above and the subjects were randomized in a 1:1:1 ratio to Maraviroc once daily, Maraviroc twice daily, or placebo. No increased risk of infection or HIV-1 disease progression was observed in the subjects who received Maraviroc. Use of Maraviroc was not associated with a significant decrease in HIV-1 RNA compared with placebo in these subjects and no adverse effect on CD4+ cell count was noted.
Trial in Treatment-Naive Subjects
Trial A4001026 was a randomized, double-blind, multicenter trial in subjects infected with CCR5-tropic HIV-1 classified by the original TROFILE tropism assay. Subjects were required to have plasma HIV-1 RNA greater than or equal to 2,000 copies per mL and could not have: 1) previously received any antiretroviral therapy for greater than 14 days, 2) an active or recent opportunistic infection or a suspected primary HIV-1 infection, or 3) phenotypic or genotypic resistance to zidovudine, lamivudine, or efavirenz. Subjects were randomized in a 1:1:1 ratio to Maraviroc 300 mg once daily, Maraviroc 300 mg twice daily, or efavirenz 600 mg once daily, each in combination with lamivudine/zidovudine. The efficacy and safety of Maraviroc are based on the comparison of Maraviroc twice daily versus efavirenz. In a pre-planned interim analysis at 16 weeks, Maraviroc 300 mg once daily failed to meet the pre-specified criteria for demonstrating non-inferiority and was discontinued.
The demographic and baseline characteristics of the maraviroc and efavirenz treatment groups were comparable (Table 18). Subjects were stratified by screening HIV-1 RNA levels and by geographic region. The median CD4+ cell counts and mean HIV-1 RNA at baseline were similar for both treatment groups.
Table 18. Demographic and Baseline Characteristics of Subjects in Trial A4001026 Maraviroc
300 mg Twice Daily +Lamivudine/Zidovudine (n = 360)
Efavirenz
600 mg Once Daily +Lamivudine/Zidovudine (n = 361)
Age (years): Mean 36.7 37.4 Range 20-69 18-77 Female, n% 104 (29) 102 (28) Race, n%: White 204 (57) 198 (55) Black 123 (34) 133 (37) Asian 6 (2) 5 (1) Other 27 (8) 25 (7) Median (range) CD4+ cell count (cells/microL) 241 (5-1,422) 254 (8-1,053) Median (range) HIV-1 RNA (log10 copies/mL) 4.9 (3-7) 4.9 (3-7) The treatment outcomes at 96 weeks for Trial A4001026 are shown in Table 19. Treatment outcomes are based on reanalysis of the screening samples using a more sensitive tropism assay, enhanced sensitivity TROFILE HIV tropism assay, which became available after the Week 48 analysis; approximately 15% of the subjects identified as CCR5-tropic in the original analysis had dual/mixed- or CXCR4-tropic virus. Screening with enhanced sensitivity version of the TROFILE tropism assay reduced the number of maraviroc virologic failures with CXCR4- or dual/mixed-tropic virus at failure to 12 compared with 24 when screening with the original TROFILE HIV tropism assay.
Table 19. Trial Outcome (Snapshot) at Week 96 Using Enhanced Sensitivity Assay*
Outcome at Week 96 †Maraviroc 300 mg Twice
Daily +
Lamivudine/Zidovudine (n = 311)
n (%)Efavirenz
600 mg Once Daily + Lamivudine/Zidovudine (n = 303)
n (%)Virologic Responders:
(HIV-1 RNA <400 copies/mL)199 (64) 195 (64) Virologic Failure: Non-sustained HIV-1 RNA
suppression39 (13) 22 (7) HIV-1 RNA never suppressed 9 (3) 1 (<1) Virologic Responders:
(HIV-1 RNA <50 copies/mL)
183 (59)190 (63) Virologic Failure:
Non-sustained HIV-1 RNA 43 (14) 25 (8) HIV-1 RNA never suppressed 21(7) 3(1) Discontinuations due to: Adverse events 19 (6) 47 (16) Death
Other ‡2 (1)
43 (14)2 (1)
36 (12)*The total number of subjects (311, 303) in Table 19 represents the subjects who had a CCR5-tropic virus in the reanalysis of screening samples using the more sensitive tropism assay. This reanalysis reclassified approximately 15% of subjects shown in Table 18 as having dual/mixed- or CXCR4-tropic virus. These numbers are different than those presented in Table 18 because the numbers in Table 18 reflect the subjects with CCR5-tropic virus according to the original tropism assay.
†Week 48 results: Virologic responders (less than 400): 228 of 311 (73%) in Maraviroc, 219 of 303 (72%) in efavirenz; Virologic responders (less than 50): 213 of 311 (69%) in Maraviroc, 207 of 303 (68%) in efavirenz.
‡Other reasons for discontinuation include lost to follow-up, withdrawn, protocol violation, and other.
The median increase from baseline in CD4+ cell counts at Week 96 was 184 cells per mm3 for the arm receiving Maraviroc compared with 155 cells per mm3 for the efavirenz arm.
14.2 Clinical Studies in Pediatric Subjects
Trial in CCR5-Tropic, Treatment-Experienced Subjects
Trial A4001031 is an open-label, multicenter trial in pediatric subjects aged 2 to less than 18 years infected with only CCR5‑tropic HIV‑1. Subjects were required to have HIV‑1 RNA greater than 1,000 copies per mL at screening. All subjects (n = 103) received Maraviroc twice daily and OBT. Dosing of Maraviroc was based on BSA and doses were adjusted based on whether the subject was receiving potent CYP3A inhibitors and/or inducers.
The population was 52% female and 69% black, with mean age of 10 years (range: 2 to 17 years). At baseline, mean plasma HIV-1 RNA was 4.4 log10 copies per mL (range: 2.4 to 6.2 log10 copies per mL), mean CD4+ cell count was 551 cells per mm3 (range: 1 to 1,654 cells per mm 3), and mean CD4+ percent was 21% (range: 0% to 42%).
At 48 weeks, 48% of subjects treated with Maraviroc and OBT achieved plasma HIV-1 RNA less than 48 copies per mL and 65% of subjects achieved plasma HIV-1 RNA less than 400 copies per mL. The mean CD4+ cell count (percent) increase from baseline to Week 48 was 247 cells per mm 3(5%).
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Maraviroc film-coated tablets are available as follows:
150-mg, and 300-mg tablets are blue, oval, film-coated tablets, debossed “I3” on one side and “24” and “25”, respectively, on the other side.
150-mg tablets: Bottle of 60 tablets (NDC: 72319-024-02).
300-mg tablets: Bottle of 60 tablets (NDC: 72319-025-02).
Maraviroc film‑coated tablets should be stored at 20 oC to 25 oC (68 oF to 77 oF); excursions permitted between 15 oC and 30 oC (59 oF and 86 oF) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Hepatotoxicity
Inform patients that hepatotoxicity, including life-threatening cases, has been reported with Maraviroc; therefore, it is important to inform the healthcare professional if patients have underlying hepatitis B or C or elevations in liver-associated tests prior to treatment. Inform patients to stop Maraviroc and seek medical evaluation immediately if they develop signs or symptoms of hepatitis or allergic reaction following use of Maraviroc. Advise patients that laboratory tests for liver enzymes and bilirubin will be ordered prior to starting Maraviroc, at other times during treatment, and if they develop severe rash or signs and symptoms of hepatitis or an allergic reaction on treatment [see Dosage and Administration (2.1), Warnings and Precautions (5.1, 5.2)].
Cardiovascular Events
When administering Maraviroc in patients with cardiovascular comorbidities, a history of postural hypotension or receiving concomitant medication known to lower blood pressure, advise patients that they may be at increased risk for cardiovascular events. Advise patients to avoid driving or operating machinery if they experience dizziness while taking Maraviroc [see Warnings and Precautions (5.3)].
Drug Interactions
Advise patients to inform their healthcare provider of concomitant HIV medications as dosage of Maraviroc may be modified depending on other HIV medications taken with Maraviroc. Advise patients that coadministration of Maraviroc with St. John’s wort is not recommended as it can lead to loss of virologic response and possible resistance to Maraviroc [see Dosage and Administration (2.2), Drug Interactions (7.1)].
Missed Dosage
Inform patients that it is important to take Maraviroc in combination with other antiretroviral medications on a regular dosing schedule with or without food. Advise patients to avoid missing doses as it can result in development of resistance. Instruct patients that if they miss a dose, to take it as soon as they remember. Advise patients not to double their next dose or take more than the prescribed dose [see Dosage and Administration (2.2)].
Pregnancy
Inform patients that there is insufficient data on the safety of Maraviroc in pregnancy. Inform patients that there is an antiretroviral pregnancy registry that monitors pregnancy outcomes in women exposed to Maraviroc during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)].
COMBIVIR is a trademark of its respective owner and is not a trademark of i3 Pharmaceuticals, LLC.
TROFILE is a trademark owned by or licensed to Monogram BioSciences, Inc., and is not owned by or licensed to i3 Pharmaceuticals, LLC. The maker of this brand is not affiliated with and does not endorse i3 Pharmaceuticals’ or its products.
Manufactured & distributed by:
i3 Pharmaceuticals, LLC
Warminster, PA, 18974
USAOS025-02 REV.0823
Revised: August 2023PHARMACIST‑DETACH HERE AND GIVE MEDICATION GUIDE TO PATIENT
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _
-
MEDICATION GUIDE
MEDICATION GUIDE
Maraviroc (mə-RAV-i-rok) tabletsWhat is the most important information I should know about Maraviroc?
Maraviroc can cause serious side effects including serious liver problems (liver toxicity). Some people who take Maraviroc can develop a severe rash or an allergic reaction before liver problems happen and may be life-threatening. Stop taking Maraviroc and call your healthcare provider right away if you get any of the following signs or symptoms of liver problems:- an itchy rash on your body (allergic reaction)
- your skin or the white part of your eyes turns yellow (jaundice)
- dark or “tea-colored” urine
- vomiting
- pain, aching, or tenderness on the right side of your stomach area
Your healthcare provider will do blood tests to check your liver before you begin treatment with Maraviroc and as needed during treatment with Maraviroc. What are Maraviroc tablets? Maraviroc tablet is a prescription Human Immunodeficiency Virus-1 (HIV-1) medicine given with other HIV-1 medicines to treat CCR5-tropic HIV-1 infection in adults and children 2 years of age and older weighing at least 22 lb (10 kg). HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
Use of Maraviroc is not recommended in people with dual/mixed- or CXCR4‑tropic HIV‑1.
Maraviroc tablets should not be used in children weighing less than 22 pounds (10 kg).Do not take Maraviroc if youhave severe kidney problems or are on hemodialysis and are also taking certain other medications. Before you take Maraviroc, tell your healthcare provider about all of your medical conditions, including if you:
have or have had liver problems including hepatitis B or C virus infection.
have heart problems.
have kidney problems.
have low blood pressure or take medicines to lower blood pressure.
are pregnant or plan to become pregnant. It is not known if Maraviroc may harm your unborn baby.
Pregnancy Registry. There is a pregnancy registry for women who take Maraviroc during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
are breastfeeding or plan to breastfeed. Do not breastfeed if you take Maraviroc. You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby. Talk to your healthcare provider about the best way to feed your baby.Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines may interact with Maraviroc. Keep a list of your medicines to show your healthcare provider and pharmacist.
You can ask your healthcare provider or pharmacist for a list of medicines that interact with Maraviroc.
Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Maraviroc with other medicines. Your healthcare provider may need to change your dose of Maraviroc when you take it with certain medicines. You should not take Maraviroc if you also take St. John’s wort ( Hypericum perforatum). How should I take Maraviroc tablets?
Take Maraviroc exactly as your healthcare provider tells you.
Do not change your dose or stop taking Maraviroc without first talking with your healthcare provider.
If you miss a dose of Maraviroc, take it as soon as you remember. Do not take 2 doses at the same time. If you are not sure about your dosing, call your healthcare provider.
Stay under the care of a healthcare provider during treatment with Maraviroc.
Swallow Maraviroc tablets whole. Do not chew the tablets.
Maraviroc may be taken with or without food.
Your healthcare provider will prescribe a dose of Maraviroc based on your child’s body weight and other medicines they are taking.
Tell your healthcare provider if your child has trouble swallowing tablets. Maraviroc comes as tablets or as a liquid (oral solution).
Do not run out of Maraviroc. The virus in your blood may increase and the virus in your blood may become harder to treat. When your supply starts to run low, get more from your healthcare provider or pharmacy.
If you take too much Maraviroc, call your healthcare provider or go to the nearest hospital emergency room right away.What are the possible side effects of Maraviroc?
Maraviroc can cause serious side effects including:See “What is the most important information I should know about Maraviroc?” Severe skin rash and allergic reactions. Severe and potentially life-threatening
skin reactions and allergic reactions have been reported in some people taking Maraviroc. If you develop a rash with any of the following symptoms, stop using Maraviroc and contact your healthcare provider right away:- fever
- generally ill feeling
- muscle aches
- blisters or sores in your mouth
- blisters or peeling of the skin
- redness or swelling of the eyes
- swelling of the mouth or face or lips
- problems breathing
- yellowing of the skin or whites of your eyes
- dark or tea-colored urine
- pain, aching, or tenderness on the right side below the ribs
- loss of appetite
- nausea/vomiting
Heart problemsincluding heart attack.
Low blood pressure when standing up(postural hypotension)that can cause dizziness or fainting. You should avoid driving or operating heavy machinery if you have dizziness during treatment with Maraviroc.
Changes in your immune system (Immune Reconstitution Syndrome)can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you develop new symptoms during treatment with Maraviroc.
Possible chance of infection or cancer.Maraviroc affects other immune system cells and therefore may possibly increase your chance for getting other infections or cancer.The most common side effects of Maraviroc in adults include coldsand cold- like symptoms, cough, fever, rash, bloating and gas, indigestion, constipation, and dizziness.
The most common side effects of Maraviroc in children includevomiting, abdominal pain, diarrhea, nausea, and dizziness.
These are not all the possible side effects of Maraviroc. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.How should I store Maraviroc?
Store Maraviroc tablets at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Maraviroc and all medicines out of the reach of children.
General information about the safe and effective use of Maraviroc Medicines are sometimes prescribed for purposes other than those mentioned in a Medication Guide. Do not use Maraviroc for a condition for which it was not prescribed. Do not give Maraviroc to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for the information about Maraviroc that is written for health professionals.What are the ingredients in Maraviroc? Active ingredient: maraviroc Inactive ingredients:
Tablets: Dibasic calcium phosphate (anhydrous), magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. Tablet film-coating contains: FD&C blue # 2 aluminum lake, soya lecithin, polyethylene glycol (macrogol 3350), polyvinyl alcohol, talc, and titanium dioxide.Manufactured and distributed by:
i3 Pharmaceuticals, LLC
200 Park Avenue
Warminster, PA 18974This Medication Guide has been approved by the U.S. Food and Drug Administration.
OS025-02 REV.0823
Revised: 08/2023 -
PRINCIPAL DISPLAY PANEL - 150 mg Tablet Bottle Label
NDC: 72319-024-02
Maraviroc Tablets
150 mg
ALWAYS DISPENSE WITH MEDICATION GUIDE
60 Tablets
Rx only
i3 Pharmaceuticals, LLC

-
PRINCIPAL DISPLAY PANEL
NDC: 72319-025-02
Maraviroc Tablets
300 mg
ALWAYS DISPENSE WITH MEDICATION GUIDE
60 Tablets
Rx only
i3 Pharmaceuticals, LLC

-
INGREDIENTS AND APPEARANCE
MARAVIROC
maraviroc tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72319-024 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MARAVIROC (UNII: MD6P741W8A) (MARAVIROC - UNII:MD6P741W8A) MARAVIROC 150 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score no score Shape OVAL Size 16mm Flavor Imprint Code I3;24 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72319-024-02 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217114 09/15/2023 MARAVIROC
maraviroc tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72319-025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MARAVIROC (UNII: MD6P741W8A) (MARAVIROC - UNII:MD6P741W8A) MARAVIROC 300 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score no score Shape OVAL Size 19mm Flavor Imprint Code I3;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72319-025-02 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/15/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217114 09/15/2023 Labeler - i3 Pharmaceuticals, LLC (080127275) Registrant - i3 Pharmaceuticals, LLC (080127275) Establishment Name Address ID/FEI Business Operations i3 Pharmaceuticals, LLC 080127275 manufacture(72319-024, 72319-025) , pack(72319-024, 72319-025)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.