Sodium Bicarbonate 650 mg
Sodium Bicarbonate by
Drug Labeling and Warnings
Sodium Bicarbonate by is a Otc medication manufactured, distributed, or labeled by TWIN MED LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SODIUM BICARBONATE- sodium bicarbonate tablet
TWIN MED LLC
----------
Sodium Bicarbonate 650 mg
Warnings
Do not usethis product if you are on a sodium-restricted diet unless directed by a doctor. Do not take more than 24 tablets for adults up to 60 years of age (or 12 tablets for adults 60 years of age and older) in a 24 hour period nor use maximum dosage for more than 2 weeks, except under the advice and supervision of a physician. As with any drug, if you are pregnant or nursing a baby, seek advice of a health professional before using this product.
Stomach Warning
TO AVOID SERIOUS INJURY, DO NOT TAKE UNTIL TABLET IS COMPLETELY DISSOLVED IT IS VERY IMPORTANT NOT TO TAKE THIS PRODUCT WHEN OVERLY FULL FROM FOOD OR DRINK.
Consult a doctor if severe stomach pain occurs after taking this product.
Directions
Adults
Take 1 tablet, dissolved in a glass of water, as needed. Maximum daily dose for adults up to 60 years of age is 24 tablets. Maximum daily dose for adults 60 years of age or older is 12 tablets. Dissolve completely in water before drinking.
DO NOT EXCEED RECOMMENDED DOSE.Not recommended for children.
Other information
- each tablet contains: sodium 178 mg (7.74 mEq)
- store at room temperature 15º-30ºC (59º-86°F) in a well-closed container as defined in the USP
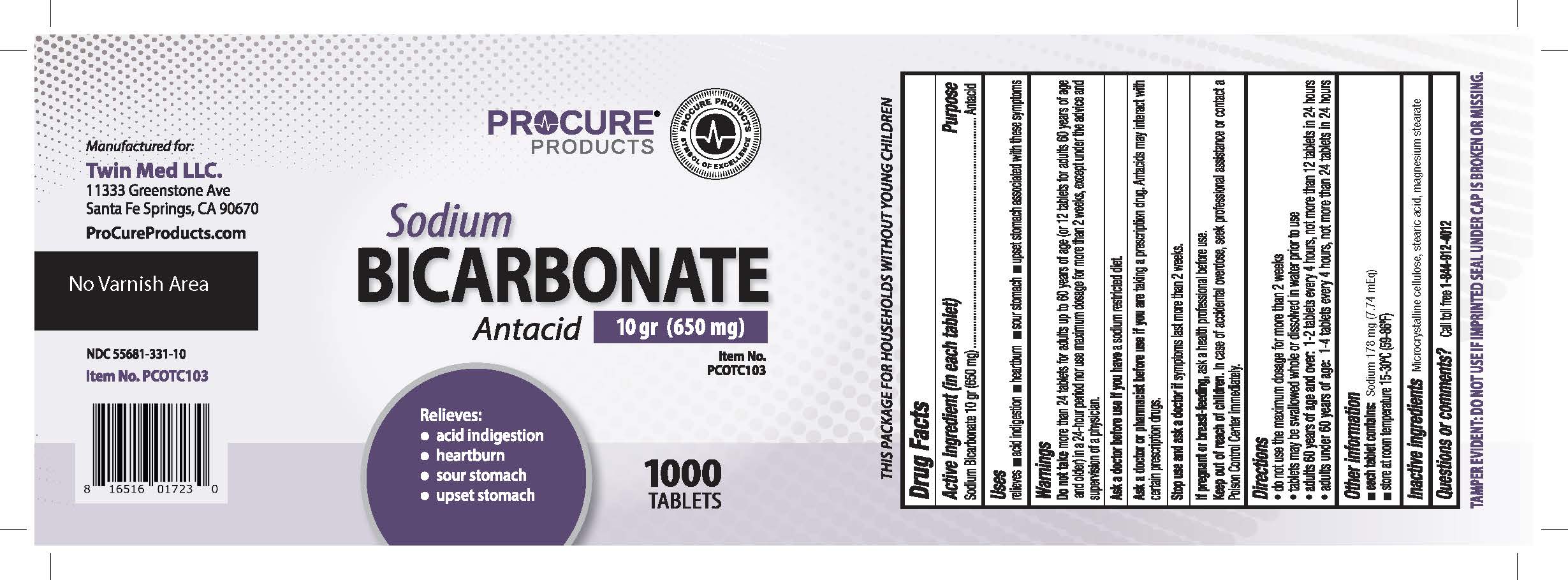
PRINCIPAL DISPLAY PANEL - 1000 Tablet Bottle Label
NDC: 55681-331-10
Sodium Bicarbonate
Tablets, USP
10 gr (650 mg)
ANTACID
| SODIUM BICARBONATE
sodium bicarbonate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - TWIN MED LLC (009579330) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ELYSIUM PHARMACEUTICALS LIMITED | 915664486 | manufacture(55681-331) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.