Pureskin Liquid Wart Corn Removal Mask by Dr.luke Healthcare LLC 83176-005 Completed
Pureskin Liquid Wart Corn Removal Mask by
Drug Labeling and Warnings
Pureskin Liquid Wart Corn Removal Mask by is a Otc medication manufactured, distributed, or labeled by Dr.luke Healthcare LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
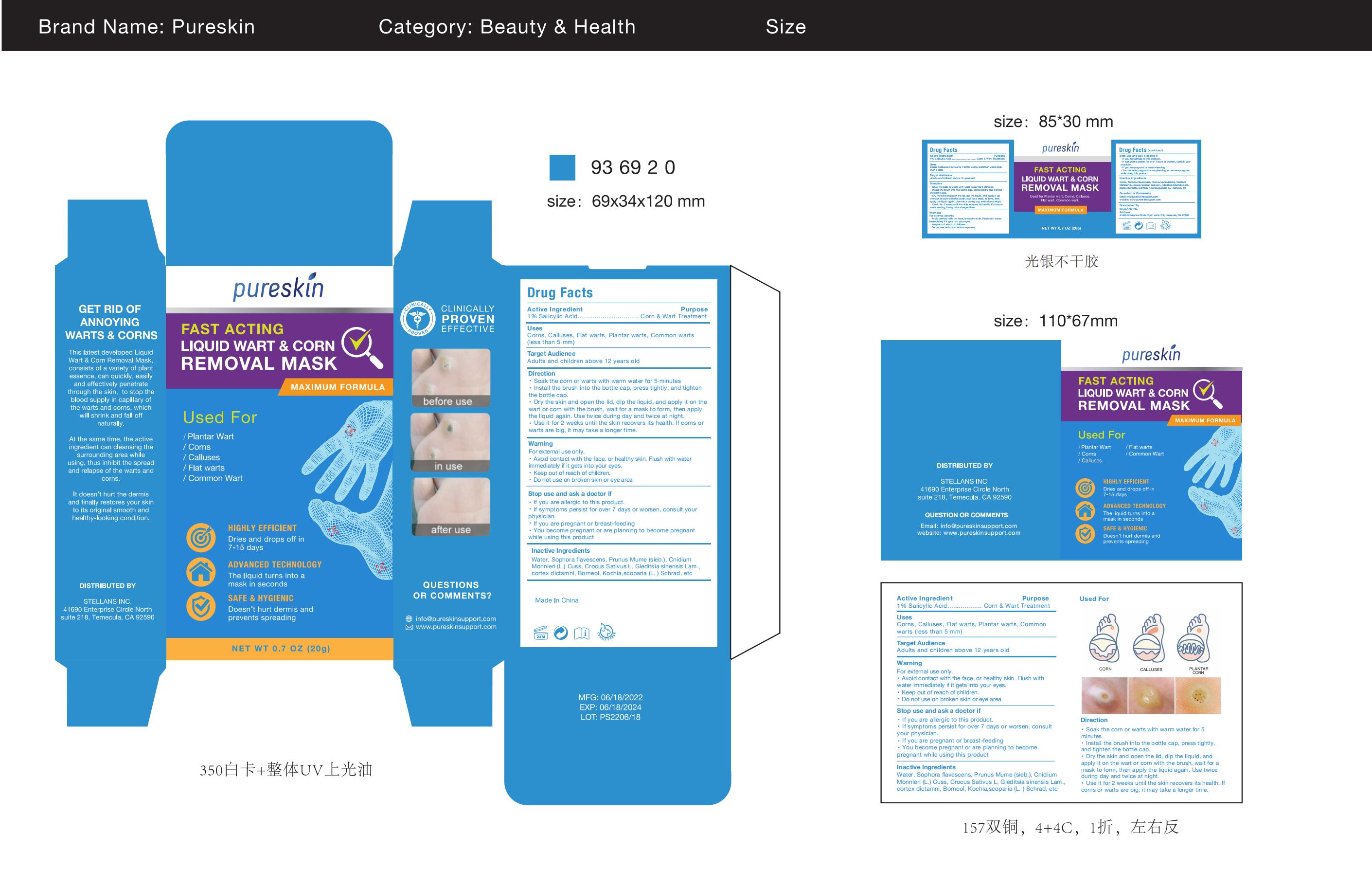
PURESKIN LIQUID WART CORN REMOVAL MASK- liquid wart corn removal mask paste
Dr.luke Healthcare LLC
----------
83176-005 Completed
Warnings
For external use only.Avoid contact with the face, or healthy skin. Flush with waterimmediately if it gets into your eyes.Keep out of reach of children.Do not use on broken skin or eye area
WHEN USING SECTION
For external use only.Avoid contact with eyes, flush with water immediately if it getsinto your eyes.Keep out of reach of children.
STOP USE section
If you are allergic to this product.If symptoms persist for over 7 days or worsen, consult yourphysician. If you are pregnant or breast-feeding.You become pregnant or are planning to become pregnantwhile using this product
ASK DOCTOR
If you are allergic to this product.If symptoms persist for over 7 days or worsen, consult yourphysician. If you are pregnant or breast-feeding.You become pregnant or are planning to become pregnantwhile using this product
Directions
Soak the corn or warts with warm water for 5 minutesInstall the brush into the bottle cap, press tightly, and tightenthe bottle cap.
Dry the skin and open the lid, dip the liquid, and apply it on thewart or corn with the brush, wait for a mask to form, then applythe liquid again. Use twice during day and twice at night.Use it for 2 weeks until the skin recovers its health. lf corns orwarts are big, it may take a longer time.
| PURESKIN LIQUID WART CORN REMOVAL MASK
liquid wart corn removal mask paste |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Dr.luke Healthcare LLC (118868014) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dr.luke Healthcare LLC | 118868014 | manufacture(83176-005) , label(83176-005) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.