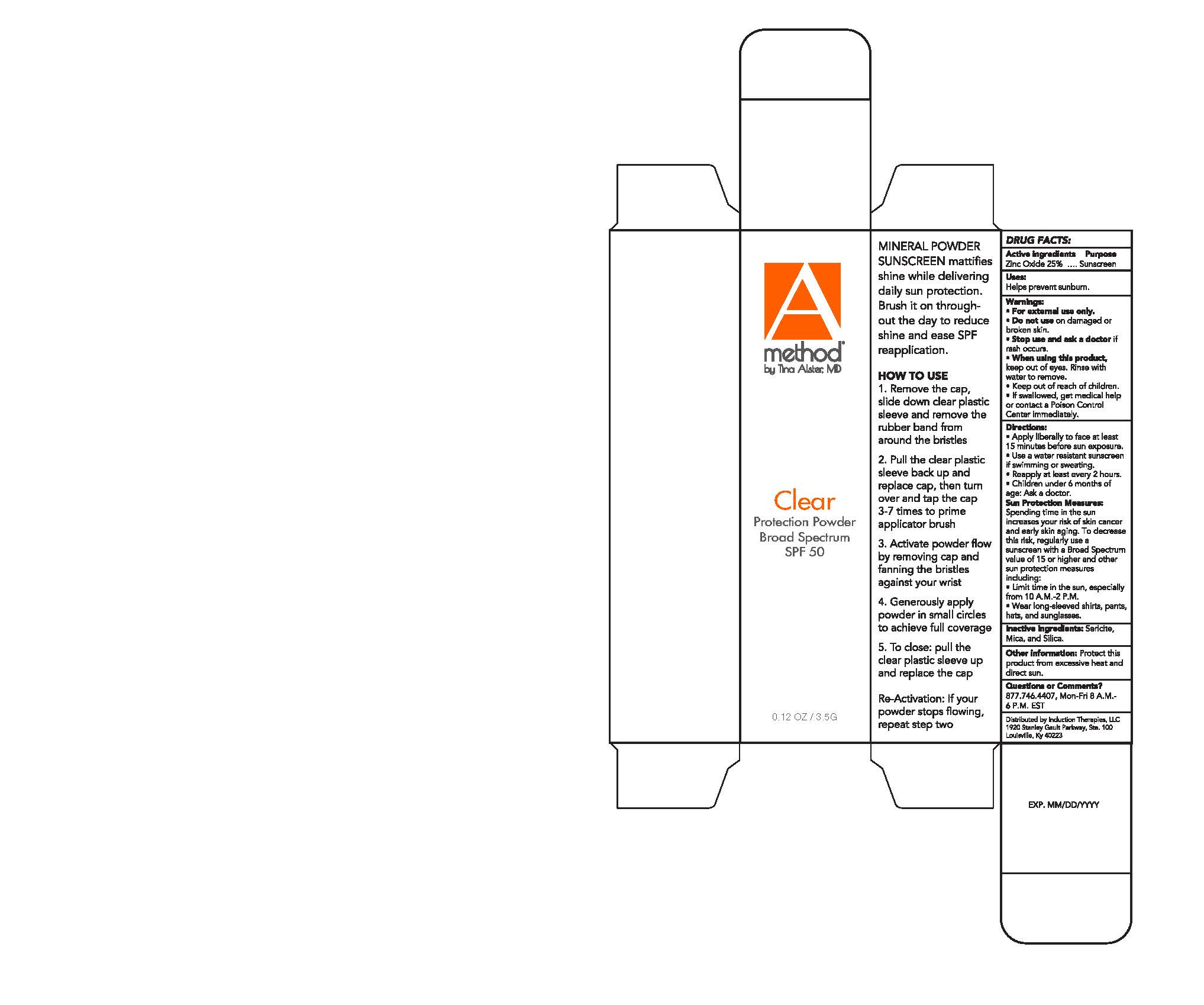
A Method Clear Protection Powder 3.5g
A Method Clear Protection Powder 3.5g by
Drug Labeling and Warnings
A Method Clear Protection Powder 3.5g by is a Otc medication manufactured, distributed, or labeled by Induction Therapies LLC, Induction Therapies, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
A METHOD CLEAR PROTECTION POWDER 3.5G- zinc oxide powder
Induction Therapies LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
A Method Clear Protection Powder 3.5g
Side Panel Box
MINERAL POWDER SUNSCREEN mattifies shine while delivering daily sun protection. Brush it on throughout the day to reduce shine and ease SPF reapplication.
HOW TO USE
1. Remove the cap, slide down clear plastic sleeve and remove the rubber band from around the bristles
2. Pull the clear plastic sleeve back up and replace cap, then turn over and tap the cap 3-7 times to prime applicator brush
3. Activate powder flow by removing cap and fanning the bristles against your wrist
4. Generously apply powder in small circles to achieve full coverage
5. To close: pull the clear plastic sleeve up and replace the cap
Re-Activation: If your powder stops flowing, repeat step two
Drug Facts Box
DRUG FACTS:
Warnings
Warnings:
For external use only.
Do not use on damaged or broken skin.
Stop use and ask a doctor if rash occurs.
When using this product, keep out of eyes. Rinse with water to remove.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Directions:
Apply liberally to face at least 15 minutes before sun exposure.
Use a water resistant sunscreen if swimming or sweating.
Reapply at least every 2 hours.
Children under 6 months of age: Ask a doctor.
Sun Protection Measures
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m.-2 p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses.
Tube Labeling
Uses: Helps prevent sunburn.
Warnings: For external use only. Do not use on damaged or broken skin. Stop use and ask a doctor if rash occurs. When using this product, keep out of eyes. Rinse with water to remove. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions: Apply liberally to face at least 15 minutes before sun exposure. Use a water resistant sunscreen if swimming or sweating. Reapply at least every 2 hours. Children under 6 months of age: Ask a doctor.
Active Ingredients: Zinc Oxide 25%
Inactive Ingredients: Sericite, Mica, and Silica
Questions or Comments? 877.746.4407, Mon-Fri 8A.M.-6P.M. EST
See outer box for all Drug Facts
| A METHOD CLEAR PROTECTION POWDER 3.5G
zinc oxide powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Induction Therapies LLC (098256562) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Induction Therapies, LLC | 098256562 | label(81846-003) | |