Eye Itch Relief by Cardinal Health / Akorn, Inc. / Akorn, Inc
Eye Itch Relief by
Drug Labeling and Warnings
Eye Itch Relief by is a Otc medication manufactured, distributed, or labeled by Cardinal Health , Akorn, Inc., Akorn, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
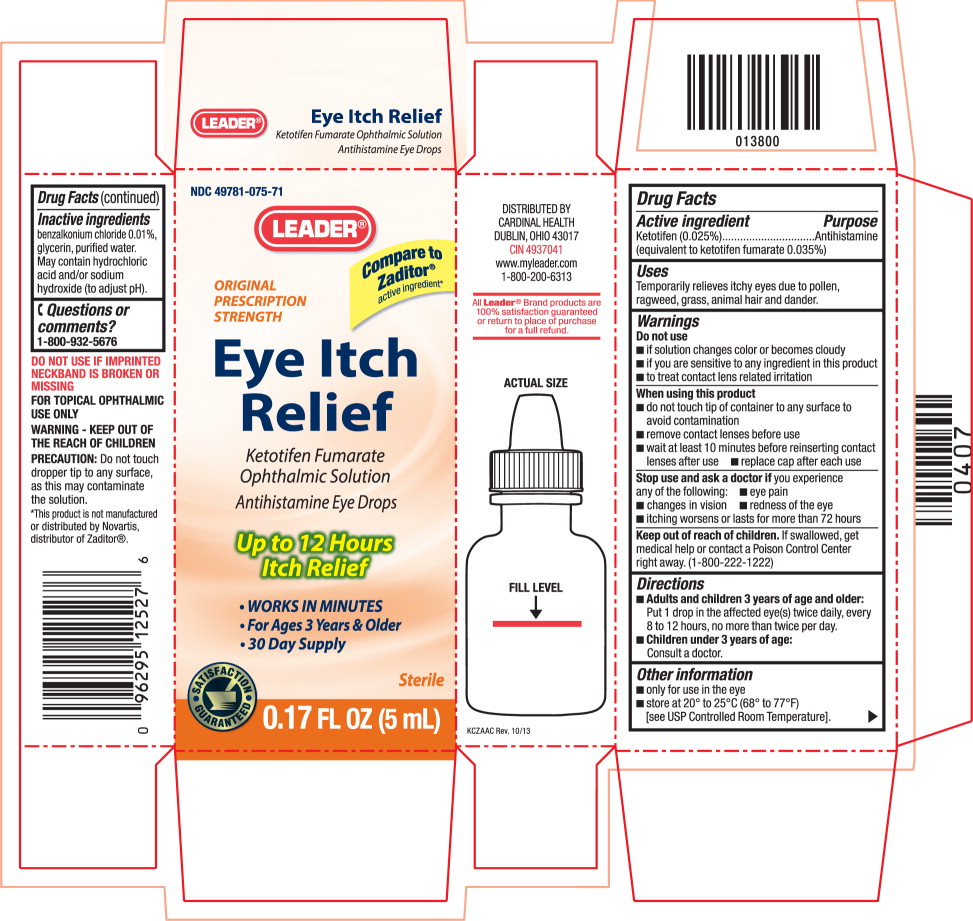
EYE ITCH RELIEF- ketotifen fumarate solution/ drops
Cardinal Health
----------
Warnings
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
Directions
-
Adults and children 3 years of age and older:
Put 1 drop in the affected eye(s) twice daily, every 8 to 12 hours, no more than twice per day. -
Children under 3 years of age:
Consult a doctor.
Other information
- only for use in the eye
- store at 20° to 25°C (68° to 77°F)
[see USP Controlled Room Temperature].
Inactive ingredients
benzalkonium chloride 0.01%, glycerin, purified water.
May contain hydrochloric acid and/or sodium hydroxide (to adjust pH).
Principal Display Panel Text for Container Label:
NDC: 49781-075-71
LEADER Logo®
ORIGINAL PRESCRIPTION STRENGTH
Eye Itch Relief
Ketotifen Fumarate Ophthalmic Solution
Antihistamine Eye Drops
Sterile
0.17 FL OZ (5 mL)
Principal Display Panel Text for Carton Label:
NDC: 49781-075-71
LEADER Logo®
Compare to
Zaditor®
active ingredient*
ORIGINAL
PRESCRIPTION
STRENGTH
Eye Itch
Relief
Ketotifen Fumarate
Ophthalmic Solution
Antihistamine Eye Drops
Up to 12 Hours
Itch Relief
- WORKS IN MINUTES
- For Ages 3 Years & Older
- 30 Day Supply
Sterile
SATISFACTION
GUARANTEED Logo
0.17 FL OZ (5 mL)
| EYE ITCH RELIEF
ketotifen fumarate solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cardinal Health (097537435) |
 Questions or comments?
Questions or comments?