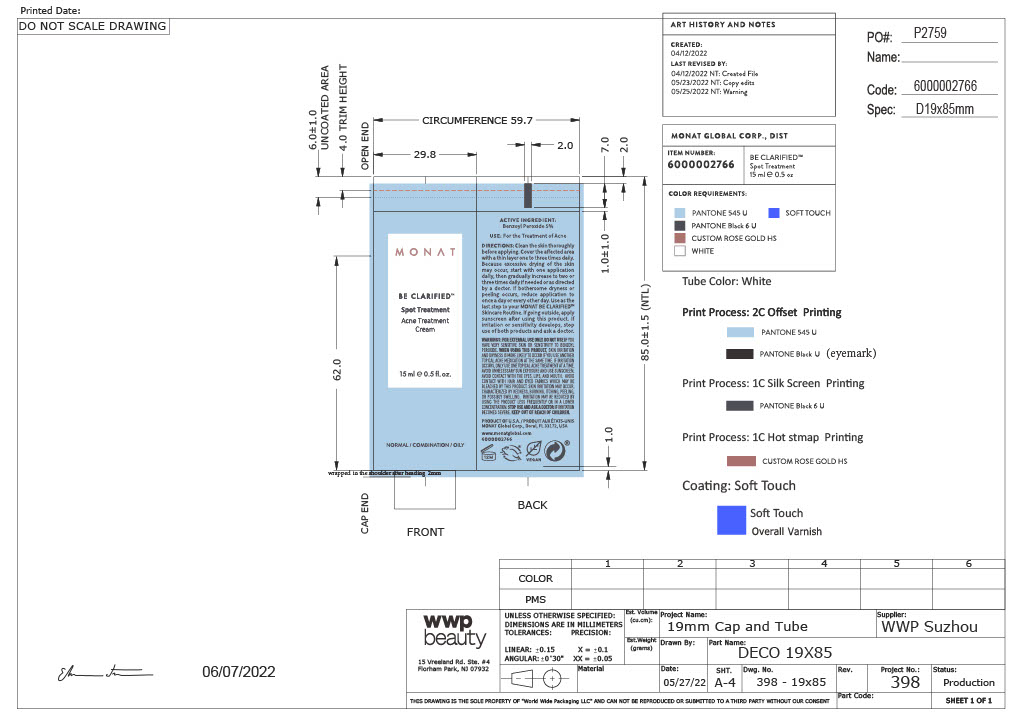
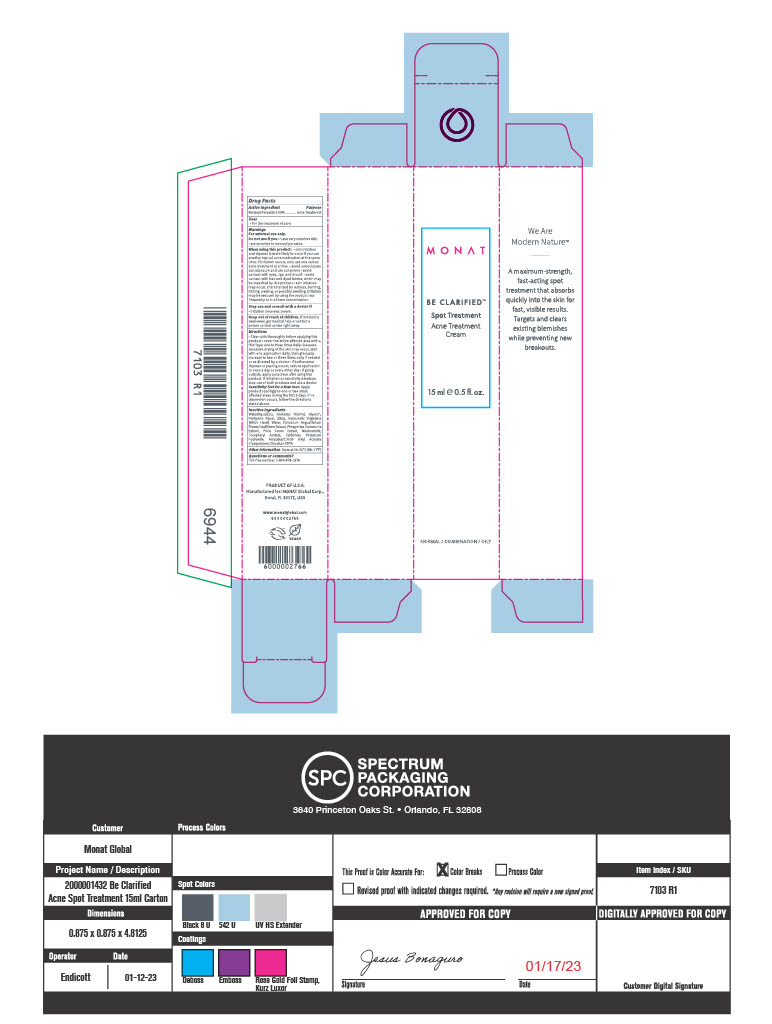
MONAT Be Clarified Spot Treatment
Acne Spot Treatment by
Drug Labeling and Warnings
Acne Spot Treatment by is a Otc medication manufactured, distributed, or labeled by MONAT GLOBAL CORP, Dermacare MFG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ACNE SPOT TREATMENT- benzoyl peroxide cream
MONAT GLOBAL CORP
----------
MONAT Be Clarified Spot Treatment
For external use only
When using this product
- skin irritation and dryness are more likely to occur if you use another topicalk acne medication at the same time. If irritation occurs, only use one topical acne treatment at a time.
- avoid unnecessary sun exposure and use sunscreen
- avoid contact with eyes, lips, and mouth.
- avoid contact with hair and dyed fabrics, which may be bleached by this product.
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
Directions
Directions
- Clean skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside. apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and sak a doctor.
Sensitivity Test for a new User. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
Inactive Ingredients
Water, Isostearyl Alcohol, Glycerin, Pentylene Glycol, Silica, Hamamelis Virginiana (Witch Hazel) Water, Epilobium Angustifolium Flower/Leaf/Stem Extract, Phragmites Communis Extract, Poria Cocos Extract, Niacinamide, Tocopheryl Acetate, Carbomer, Potassium Hydroxide, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Disodium EDTA.
| ACNE SPOT TREATMENT
benzoyl peroxide cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - MONAT GLOBAL CORP (027036949) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dermacare MFG | 116817470 | manufacture(78518-013) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.